Primary ammonium/tertiary amine-mediated controlled ring opening polymerisation of amino acid N-carboxyanhydrides.
Reads0
Chats0
TLDR
Stable commercial primary ammonium chlorides were combined with tertiary amines to initiate the controlled ring opening polymerisation of amino acid N-carboxyanhydrides to yield polypeptides with defined end group structure, predetermined molar mass and narrow molarmass distribution.About:
This article is published in Chemical Communications.The article was published on 2015-10-15 and is currently open access. It has received 43 citations till now. The article focuses on the topics: Tertiary amine & Molar mass distribution.read more
Figures

Fig. 4 Time-conversion plots for the polymerisations of (a) BLG–NCA (100 equiv.) and (b) LLeu–NCA (100 equiv.) in DMF initiated by BnA HCl (1 equiv.), BnA HCl/TEA (1 : 0.5 equiv.), BnA (1 equiv.), and TEA (0.5 equiv.). Monomer conversions were determined by FT-IR spectroscopy (see ESI†); numbers in (a) are the polymer dispersities determined by SEC. 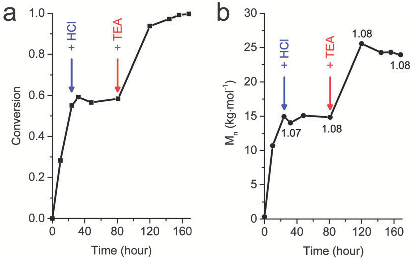
Fig. 3 Polymerisation of BLG–NCA (150 equiv.) in DMF initiated by PyA HCl/TEA (1 : 0.5 equiv.), paused at 24 h by adding HCl and resumed at 81 h by adding TEA. (a) time-conversion plot, (b) number-average molar mass and dispersities (at 24 h, 81 h, 120 h, and 168 h) determined by SEC. 
Fig. 2 SEC traces (RI in black, UV340nm in purple) of polymerisations of BLG–NCA (150 equiv.) in DMF initiated by (a) PyA HCl/TEA (1 : 1.1) at (top to bottom) 2 h, 6 h, 10 h, 24 h; (b) PyA HCl/TEA (1 : 1.5) at (top to bottom) 2 h, 6 h, 8 h, 24 h; the peak (*) is a high molar mass polystyrene (PS2M, 2 MDa) used as internal standard for calculating the monomer conversion (ESI†). 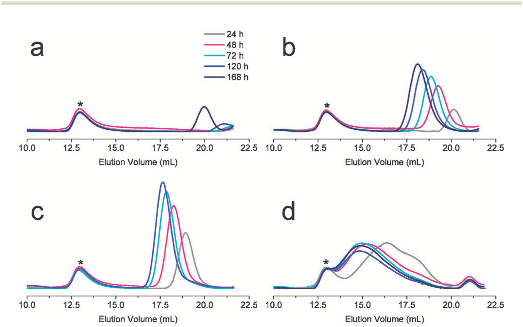
Fig. 1 SEC traces of PBLGs obtained during the polymerisations of BLG–NCA (150 equiv.) in DMF initiated by (a) TAB 3HCl (1 equiv.), r.t.; (b) TAB 3HCl (1 equiv.), 50 1C; (c) TAB 3HCl/TEA (1 : 0.5 equiv.), r.t.; and (d) TEA (0.5 equiv.), r.t.; the peak (*) is a high molar mass polystyrene (PS2M; 2 MDa) used as internal standard for calculating the monomer conversion (ESI†).
Citations
More filters
Journal ArticleDOI
High-throughput discovery of organic cages and catenanes using computational screening fused with robotic synthesis
Rebecca L. Greenaway,Valentina Santolini,Michael J. Bennison,Ben M. Alston,Chloe J. Pugh,Marc A. Little,Marcin Miklitz,E. G. B. Eden-Rump,Rob Clowes,A. Shakil,H. J. Cuthbertson,H. Armstrong,Michael E. Briggs,Kim E. Jelfs,Andrew I. Cooper +14 more
TL;DR: Computational screening with high-throughput robotic synthesis is combined to create a hybrid discovery workflow for discovering new organic cage molecules, and by extension, other supramolecular systems that form cleanly in one-pot syntheses.
Journal ArticleDOI
Ring opening polymerization of α-amino acids: advances in synthesis, architecture and applications of polypeptides and their hybrids.
Alicia Rasines Mazo,Stephanie Allison-Logan,Fatemeh Karimi,Fatemeh Karimi,Nicholas Jun-An Chan,Wenlian Qiu,Wei Duan,Neil M O'Brien-Simpson,Greg G. Qiao +8 more
TL;DR: Key architectures obtained through NCA ROP or in combination with other polymerization methods are reviewed, as these play an important role in the wide range of applications towards which polypeptides have been applied.
Journal ArticleDOI
Lithium hexamethyldisilazide initiated superfast ring opening polymerization of alpha-amino acid N -carboxyanhydrides
TL;DR: Lithium hexamethyldisilazide is used to initiate α-amino acid N-carboxyanhydride polymerizations that is very fast and can be conducted in an open vessel, and rapid synthesis of polypeptide libraries for high-throughput functional screening.
Journal ArticleDOI
Methacrylate-ended polypeptides and polypeptoids for antimicrobial and antifouling coatings
TL;DR: This dual-functional polymer brush coating can be immobilized on the surface of multiple categories of materials through the mussel-inspired pDA coating, and thus should be widely applicable for combating infection in many classes of bio-medical materials.
Journal ArticleDOI
Poly(α-l-lysine)-based nanomaterials for versatile biomedical applications: Current advances and perspectives.
Maochao Zheng,Miao Pan,Wancong Zhang,Huanchang Lin,Shenlang Wu,Chao Lu,Shijie Tang,Daojun Liu,Jianfeng Cai +8 more
TL;DR: This review aims to summarize the recent advances in PLL-based nanomaterials in these biomedical fields over the last decade by describing the synthesis of PLL and its derivatives and the main text of their recent biomedical applications and translational studies.
References
More filters
Journal ArticleDOI
Emerging applications of stimuli-responsive polymer materials
Martien A. Cohen Stuart,Wilhelm T. S. Huck,Jan Genzer,Marcus Müller,Christopher K. Ober,Manfred Stamm,Gleb B. Sukhorukov,Igal Szleifer,Vladimir V. Tsukruk,Marek W. Urban,Françoise M. Winnik,Stefan Zauscher,Igor Luzinov,Sergiy Minko +13 more
TL;DR: This work reviews recent advances and challenges in the developments towards applications of stimuli-responsive polymeric materials that are self-assembled from nanostructured building blocks and provides a critical outline of emerging developments.
Journal ArticleDOI
Nanoparticle Polymer Composites: Where Two Small Worlds Meet
TL;DR: A challenge for future studies is to create hierarchically structured composites in which each sublayer contributes a distinct function to yield a mechanically integrated, multifunctional material.
Journal ArticleDOI
Facile synthesis of block copolypeptides of defined architecture
TL;DR: A polymerization strategy that overcomes difficulties by using organonickel initiators which suppress chain-transfer and termination side reactions is described, which allows the facile synthesis of block copolypeptides with well-defined sequences, which might provide new peptide-based biomaterials with potential applications in tissue engineering, drug delivery and biomimetic composite formation.
Journal ArticleDOI
Polypeptides and 100 years of chemistry of alpha-amino acid N-carboxyanhydrides.
TL;DR: This review summarizes the literature after 1985 and reports on new aspects of the polymerization processes, such as the formation of cyclic polypeptides or novel organometal catalysts and the role of NCAs in molecular evolution on the prebiotic Earth is discussed.
Journal ArticleDOI
Synthetic polypeptides for biomedical applications
TL;DR: This article summarizes recent developments in the synthesis of copolypeptides from polymerization of α-amino acid-N-carboxyanhydrides (NCAs), focusing on their development for potential use in biomedical applications.
Related Papers (5)
Synthesis of nearly monodisperse polystyrene-polypeptide block copolymers via polymerisation of N-carboxyanhydrides.
Ivaylo Dimitrov,Helmut Schlaad +1 more