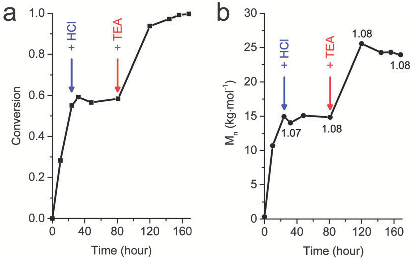
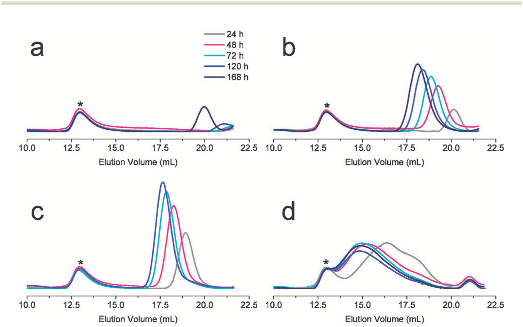
Abstract: Many natural polymeric materials (particularly structural proteins) display a hierarchy of structure over several length scales. Block copolymers are able to self-assemble into ordered nanostructures1,2, but the random-coiled nature of their polymer chains usually suppresses any further levels of organization. The use of components with regular structures, such as rigid-rod polymers, can increase the extent of spatial organization in self-assembling materials3. But the synthesis of such polymeric components typically involves complicated reaction steps that are not suitable for large-scale production. Proteins form hierarchically organized structures in which the fundamental motifs are generally α-helical coils and β-sheets4. Attempts to synthesize polypeptides with well-defined amino-acid sequences, which might adopt similar organized structures, have been plagued by unwanted side reactions5 that give rise to products with a wide range of molecular weights6,7,8,9,10, hampering the formation of well-defined peptide block copolymers11,12,13,14,15,16,17. Here I describe a polymerization strategy that overcomes these difficulties by using organonickel initiators which suppress chain-transfer and termination side reactions. This approach allows the facile synthesis of block copolypeptides with well-defined sequences, which might provide new peptide-based biomaterials with potential applications in tissue engineering, drug delivery and biomimetic composite formation.