Q2. What is the importance of the H2:CO ratio in a gasification process?
The H2:CO ratio is important for further hydrogen separation, as low values are likely to cause low bioH2 yield and high CO2 generation during water gas shift.
Q3. How could a higher CO conversion be achieved?
Close to 100% CO conversion could be achieved with higher Nickel catalyst (40-50% wt.) active at lower temperatures (180-200 ˚C), or by removing the bulk of CO2 upstream.
Q4. What is the common method of achieving the 99.95% purity required for use in fuel?
Pressure swing adsorption (PSA) is commonly employed to achieve the 99.95% purity required for use in fuel cells (Asgari et al., 2014).
Q5. What is the common type of cleaning system for small scale waste based plants?
Typical cleaning system for small scale (<100 MW) waste based plants includes tar reforming systems, dry filters (incorporating a ceramic filter unit with chemical sorbents dosing), and alkaline wet scrubbers (Zwart, 2009).
Q6. Why did the heat loss requirement require the use of electric blankets around reactors?
Because of the small-scale of the plant, heat losses necessitated the use of electric blankets around reactors to ensure components were maintained at sufficient temperature.
Q7. Why is the transition to hydrogen a longer term opportunity?
shifting completely to hydrogen offers a longer-term opportunity for bioH2 because it offers far greater carbon savings than SMR hydrogen.
Q8. What is the biggest obstacle when using these sources as feedstock?
The biggest obstacle when using these sources as feedstock is the utilization of land and clean water to produce energy crops instead of food production.
Q9. What is the key element for a consistent quantity of bioH2?
The key element for a consistent quantity of bioH2 is the production of a high quality syngas very rich in hydrogen, and suitable for catalytic processing.
Q10. What other technologies could be considered for the separation of hydrogen from gas?
Several other separation technologies could be considered, including membrane separation, physical solvents and amine systems (Granite and O’Brien, 2005; Adhikari and Fernando, 2006; Barelli et al., 2008; Shokrollahi et al., 2016).
Q11. What is the common method of delivering pure oxygen without getting to high temperature?
In order to deliver sufficient pure oxygen without getting to high temperature, oxygen/steam mixtures are typically used in practical applications.
Q12. Why are fluidised beds more suitable for small applications?
Due to their flexibility and robustness, fluidised beds are instead more suitable for small applications and for treating gross and heterogeneous feedstock (Materazzi and Lettieri, 2017b; Arena and DiGregorio, 2016).
Q13. Why is hydrogen being promoted as a potential fuel?
In recent years hydrogen has received increasing attention as a potential fuel that could be produced from non-fossil fuel sources (Hart et al., 2015; Barisano et al., 2017; Ogden, 2018), both because it can be generated with low greenhouse-gas (GHG) emissions, and because it generates no emissions at the point of use.
Q14. How much CO2 is emitted per MWh of hydrogen produced?
Assuming a commercial electrolyser efficiency to be 50 kWh/kg H2, and the same CO2 emissions associated to use of electricity, approximately 220 kg of CO2 equivalent are emitted per MWh of hydrogen produced, as also shown in (Bertuccioli et al., 2014).
Q15. What is the effect of the combination of steam-oxygen fluidised bed gasification and plasma?
The work has confirmed that the combination of steam-oxygen fluidised bed gasification and plasma refining delivers a high quality raw gas with very low levels of contaminants, while dealing at the same time with the increased amount of ashes by producing a vitrified inert product.
Q16. How was the gas composition measured in the first HTS reactor?
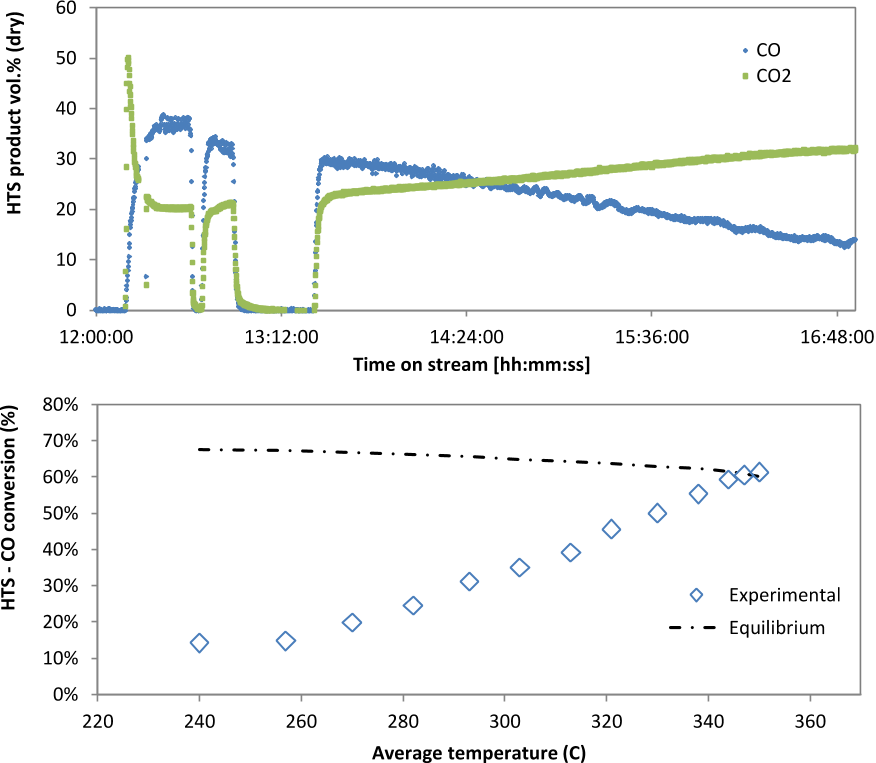
Combined flow of gas through the reactor was sufficient to give a GHSV of between 5000 and 11000 h-1.Symmetrical trends of CO and CO2 were observed in the first HTS reactor reflecting the occurrence of water gas shift as the dominating reaction from temperatures above 250°C (Figure 3).
Q17. What is the appropriate reaction condition for the examined catalysts?
It is concluded, therefore, that for the examined catalysts the most appropriate reaction condition is a H2O:CO molar ratio of approximately 2.4.2.
Q18. How many columns can be used to achieve a maximum of 93 % of hydrogen recovery?
Luberti et al. (Luberti et al., 2014) have shown that hydrogen recovery can reach a maximum of 93 % with a Polybed H2 PSA system having twelve columns.
Q19. Why is a WtH2 plant subsidised by the waste gate fees?
This is because, as for other thermochemical plants, a WtH2 plant has relatively high capital costs but operating costs are subsidised by the waste gate fees.