OATAO is an open access repository that collects the work of Toulouse
researchers and makes it freely available over the web where possible
Any correspondence concerning this service should be sent
to the repository administrator: tech-oatao@listes-diff.inp-toulouse.fr
This is an author’s version published in: http://oatao.univ-toulouse.fr/23249
To cite this version:
Candy, Laure and Vaca-Garcia, Carlos and Borredon, Marie-Elisabeth
Synthesis and characterization of oleic succinic anhydrides: Structure-property
relations. (2005) Journal of the American Oil Chemists' Society, 82 (4). 271-277.
ISSN 0003-021X
Official URL: https://doi.org/10.1007/s11746-005-1066-5

ABSTRACT: Alkenyl succinic anhydrides (ASA) were prepared
by an -ene reaction of n-alkyl (C
1
to C
5
) oleates with maleic an-
hydride. The purified compounds were characterized by FTIR,
1
H NMR, and MS analytical methods to elucidate their struc-
tures. Their physicochemical properties were systematically
studied and found to depend on the length of the alkyl radical.
Structure–property relations were established for viscosity, m.p.,
and density. The combination of a long hydrophobic chain and
a highly polar group with density values close to that of water
implied good emulsification properties for some of these mole-
cules. Comparison of the thermal properties of alkyl oleates and
their respective ASA demonstrated that the grafting of maleic
anhydride allowed the synthesis of compounds with very low
melting temperatures (less than –60°C) and good stability at
high temperatures (greater than 350°C) under both air and he-
lium atmospheres. All these properties suggest a strong poten-
tial for application in the biolubricant or surfactant fields. The
combined influences of the succinic part and variable ester moi-
eties imply that each ASA molecule has its own characteristics,
based on which applications could be developed.
K
EY WORDS: Alkenyl succinic anhydride, degradation tem-
perature, density, dynamic viscosity, fatty acid ester, maleiniza-
tion, melting temperature, renewable resources.
Alkenyl succinic anhydrides (ASA) are widely used in s
ev-
eral fields, for example, as additives in lubricants (1), inter-
mediates in organic chemistry (2), wood-preservation agents
(3), or paper-sizing agents (4,5). They are obtained by an -ene
reaction (6) between maleic anhydride (enophile) and an
alkene having an allylic hydrogen (-ene). During the reaction,
a new bond is created between two unsaturated termini, with
an allylic shift of the -ene double bond and transfer of the al-
lylic hydrogen to the enophile. Usually, the alkene is a petro-
chemical olefin, but TG or vegetable oil derivatives such as
FA also have been used; in this case, the reaction is com-
monly named maleinization (7). The -ene reaction requires a
Lewis acid and/or high temperature (>200°C) since its acti-
vation energy (E
a
) is high (>80 kJ/mol) (8). Even though the
conversion rate is improved at higher temperatures, various
side reactions can take place: enophile polymerization, alkene
oligomerization, copolymerization between the enophile and
alkene, and thermal decomposition of the ASA (retroene re-
action). Secondary products are black, waxy solids unsuitable
for the major uses of ASA.
Literature concerning the reaction between maleic anhy-
dride and unsaturated molecules can be divided in three peri-
ods. First, the reaction mechanism was studied during the
1940s and up to the early 1950s by using ASA from FA or FA
esters. Demonstration of the -ene reaction mechanism was de-
bated and finally established during this period (9–11). Sec-
ond, the strong development of petrol resources in the early
1950s favored the production of ASA from straight or
branched olefins or even from polyolefins (4,12). From then
up to the 1990s, the studies aimed at limiting secondary prod-
ucts by adding reaction catalysts, polymerization inhibitors
(8), or an aromatic solvent (13). Third, with the increased in-
terest in renewable resources clearly observed since the
1990s, new ASA have again been synthesized from vegetable
oils and their derivatives. They have essentially been used as
monomers for the fabrication of thermosets (14), as the anhy-
dride moiety can react with diamines, diols and polyols, or
epoxy resins to yield unsaturated polyester-like resins.
Among these works, only a few have been concerned with the
fundamentals of vegetable ASA compounds, such as the one
carried out by Metzger and Biermann (15), who studied the
stereochemistry of ASA obtained from methyl oleate by
1
H
and
13
C NMR. No study has been concerned with the physic-
ochemical properties of vegetable ASA compounds. Our pur-
pose was therefore to synthesize, purify, and characterize
ASA compounds obtained from oleic acid esters with differ-
ent alkyl groups (Fig. 1). The main objective was to study the
influence of ester chain length on some physicochemical
properties to establish structure–property relations useful for
defining potential fields of application. With this study, we in-
tend to favor the replacement of fossil resources by renew-
able resource-derived ASA.
EXPERIMENTAL PROCEDURES
Materials. Methyl oleate (>96%) was supplied by Fluka (St
Quentin Fallavier, France). Oleic acid and oleoyl chloride
(both technical grade >85%), as well as maleic anhydride,
ethanol, propanol, butanol, and pentanol (all >99%), were
*To whom correspondence should be addressed at Laborat
oire de Chimie Agro-
industrielle UMR 1010 INRA/INP-ENSIACET, 118 route de Narbonne; 31077
Toulouse Cedex 4, France. E-mail: Carlos.VacaGarcia@ensiacet.fr
Synthesis and Characterization of Oleic Succinic
Anh
ydrides: Structure–Property Relations
Laure Candy, Carlos Vaca-Garcia*, and Elisabeth Borredon
Laboratoire de Chimie Agro-industrielle, Unité Mixte de Recherche (UMR) 1010 Institut National de la Recherche
Agronomique (INRA)/Institut National Polytechnique de Toulouse (INP)–Ecole Nationale Supérieure des Ingénieurs en Arts
Chimiques et Technologiques (ENSIACET), 31077 Toulouse Cedex 4, France

column (silica-grafted C
18
) operated at 25°C, with a 1 mL/
min acetonitrile (Riedel-de Haën, St Quentin Fallavier,
France) flow as the mobile phase.
IR spectroscopy. IR spectra were recorded on KBr win-
dows using a Perkin-Elmer 1600 FTIR in the 400–4000 cm
–1
region.
NMR
spectroscopy.
1
H NMR spectra were obtained in a
Bruker AC200 apparatus at 200 MHz. The samples were ana-
lyzed in CDCl
3
99% (Sigma).
MS. Mass spectra were obtained in a Nermag R 1010 spec-
trometer. After dilution in CH
2
Cl
2
, the samples were chemi-
cally ionized by ammonia (NH
3
).
Density. Density was determined using a 10-mL pycnome-
ter according to ISO standard 279-1981.
Rheometry. Dynamic viscosities were measured with a
Carrimed CSL100 viscometer. The operating conditions for
oleic esters were as follows: cone angle, 2°; cone diameter, 6
cm; truncation, 56 µm; strain, constant 1 N/m
2
for 1 min; tem-
perature, 20°C. The operating conditions for ASA were as
follows: cone angle, 2°; cone diameter, 4 cm; truncation, 54
µm; strain, linear from 10 to 30 N/m
2
for 2 min; temperature,
variable from 0 to 60°C.
DSC. Thermal transitions of oleic esters were recorded on
a PerkinElmer Pyris1 apparatus. The temperature program
was –50 to 25°C at 10°C/min. ASA compounds were studied
in a Netzsch 204 apparatus. The temperature program was
−120 to 25°C at 10°C/min. Melting points of these com-
pounds were calculated from thermograms according to the
tangent method.
Thermogravimetrical analysis. The degradation tempera-
tures of ASA corresponding to 10 and 50% mass loss were de-
termined in a Setaram TG-DTG 92 apparatus. The samples were
heated from 20 to 600°C at 10°C/min under air and helium.
RESULTS AND DISCUSSION
Synthesis of alkyl oleates. Five oleic acid alkyl esters, from
methyl to pentyl, with reasonably high purity (>85%) were
required for ASA synthesis. Methyl oleate was commercially
available. Butyl oleate and pentyl oleate were successfully
synthesized by the esterification technique. After distillation,
no residual oleic acid accompanied the desired product. The
purities of these alkyl oleates were 90 and 87%, respectively,
the rest being butyl and pentyl esters of other FA as the initial
source, which were of technical grade. On the contrary, the
esterification technique was not successful for the synthesis
purchased from Sigma (St Quentin Fallavier, France). 4-
Toluene-sulfonic acid (p-T
SA, >99%) was supplied by Fluka.
All reagents were used as received without further purification.
Preparation of butyl and pentyl oleates. The syntheses of
butyl and pentyl oleates were based on the complete esterifi-
cation of oleic acid with the corresponding alcohol. Butanol
or pentanol (2 mol), oleic acid (1 mol), and p-TSA (0.1%
based on total weight) were placed in a 500-mL three-necked
reactor and heated to 95°C. The reactor was equipped with a
magnetic stirrer, a thermometer, and a distillation column fol-
lowed by a condenser. The azeotropic mixture formed by
water and alcohol was distilled off to shift the equilibrium.
Esterification was monitored by quantification of the acid
value of the medium according to ISO Standard 660:1996.
The reaction was stopped when no more azeotropic mixture
wa
s distilled and when the acidity of the medium was mini-
mal. The cooled reaction medium was washed with a NaCl-
saturated aqueous solution and then vacuum distilled. FA es-
ters were distilled between 170 and 190°C under 1 mmHg and
used immediately for the -ene reaction.
Preparation of ethyl and propyl oleates. These esters were
synthesized by acylation of the alcohol with a FA chloride.
Ethanol or propanol (1 mol) was placed in a 500-mL three-
necked reactor equipped with a nitrogen input, a mechanical
stirrer, and a c
ondenser followed by two NaOH solution
washers to trap the HCl formed. Oleoyl chloride (0.5 mol)
was added to the reactor under nitrogen at room temperature.
As the HCl evolved, the equilibrium shifted. The temperature
w
as gently raised to 90°C to complete the reaction. The
medium was then washed with a NaCl-saturated aqueous so-
lution and vacuum distilled. Ethyl and propyl oleates were
distilled between 160 and 170°C under 1 mmHg and used im-
mediately for the -ene reaction.
Synthesis and purification of ASA. Alkyl oleate (100 g) and
maleic anhydride (1.2 mol/mol of alkyl oleate) were heated at
22
0°C for 8 h under a static nitrogen atmosphere in a three-
necked reactor equipped with a magnetic stirrer and a con-
denser heated at 60°C to reflux the liquid maleic anhydride.
At the end of the reaction, the medium was vacuum distilled
(<1 mmHg). Unreacted maleic anhydride and oleic acid es-
ters were distilled at 70°C and between 160 and 190°C, re-
spectively. ASA were then distilled between 240 and 260°C
under this pressure. The purity of the distilled fractions (oleic
acid esters and ASA) was evaluated by LC. The HPLC sys-
tem was composed of a Spectra-Physics pump and a refrac-
tometer detector 350RI (Varian) at 35°C. A Spherisorb ODS2
FIG. 1. Alkenyl succinic anhydrides (ASA) compounds derived from alkyl oleates (R = –C
n
H
2n+1
, n = 1–5). Two isomers are possible.

of ethyl and propyl oleates. These reactions were not com-
plete, and significant quantities of oleic acid remained in the
reaction medium. This was probably due to the low reaction
temperature, limited by the boiling temperature of the water–
alcohol azeotrope. Moreover, the distillation of the medium
did not allow an effective separation of the ethyl and propyl
oleates from oleic acid. Consequently, the synthesis of the
former were finally properly achieved by acylation of the cor-
responding alcohol with oleoyl chloride. Purities of 84 and
89% in ethyl and propyl oleate, respectively, were reached
after distillation, with no free reagents left. The rest was as-
sumed to be ethyl or propyl esters of other FA.
Synthesis of ASA. Two isomers of ASA can be formed,
since the addition reaction of maleic anhydride on oleic acid
esters can occur either on carbon 9 or carbon 10 (atoms con-
cerned with the double bond) of the unsaturated chain. The
two isomers could not be separated by vacuum distillation,
but the HPLC analysis of the distillate showed two peaks that
were poorly resolved. They were therefore integrated as a
unique peak for purity calculations based on the following area
ratios: 95% for methyl oleate succinic anhydride (ASAOM),
99% for ethyl oleate succinic anhydride (ASAOE), 99% for
propyl oleate succinic anhydride (ASAOPr), 88% for butyl
oleate succinic anhydride (ASAOB), and 98% for pentyl oleate
succinic anhydride (ASAOPe).
ASA characterization. All distilled ASA were clear yel-
low, oily liquids (Gardner’s color index from 5 to 7). FTIR
spectra of oleic ASA presented significant bands representa-
tive of the groups present (Fig. 2). The major IR peaks aris-
ing from anhydride carbonyl stretching were located at 1785
and 1863 cm
–1
. They also showed a stretching vibration band
typ
ical of five-membered cyclic anhydrides, such as succinic
anhydride, at 917 cm
–1
. The carbonyl stretching band of the
alkyl ester appeared at 1732 cm
–1
.
1
H NMR
spectra of the samples showed the expected char-
acteristic peaks. ASA from methyl oleate showed a peak at
5.5 ppm (m, 2H) belonging to the –CH of the double bond.
The peak at 3.6 ppm (s, 3H) belonged to the –CH
3
in the ester
part. The peaks between 3.1 and 2.6 ppm (m, 3H) were as-
signed to –CH belonging to the succinic link and to the anhy-
dride moiety. The peaks at 2.2 ppm (t, 2H) and at 1.5 ppm (m,
2H), respectively, were identified as –CH
2
in positions α and
β to the ester group and part of the unsaturated chain. The
peak at 1.9 ppm (d, 2H) belonged to the –CH
2
in a position to
the double bond.
The peak at 1.2 ppm (m, 20H) was represen-
tative of the –CH
2
from the linear fatty chain. Finally, the
peak at 0.80 ppm (t, 3H) could be assigned to the terminal
–CH
3
moiety of the alkyl chain. These spectra provided con-
firmation of the nature of our products.
Additional confirmation of the structures was obtained
from MS. The M.W. of ASA compounds were obtained from
the formation of ASA–NH
4
+
ions for m/z 412, 426, 440, 454,
and 468, which corresponded to the theoretical M.W. of
ASAOM, ASAOE, ASAOPr, ASAOB, and ASAOPe. Other
characteristic peaks were m/z 395, 409, 423, 437, and 451,
corresponding to the formation of ASA–H
+
ions. For each
MS spectrum, a common peak for m/z 398 was obtained, cor-
responding to a NH
4
+
ionized fragment in which the alkyl part
of the carboxylic acid ester moiety of ASA was being re-
moved.
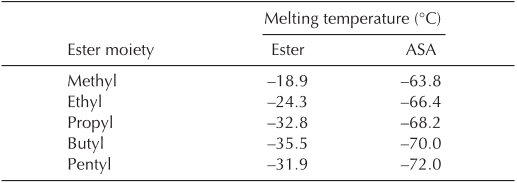
Physical properties of ASA. After confirmation of the
structure of ASA, several physical characteristics were evalu-
ated. Our conclusions took into account the stereoselectivity,
the length of the ester moiety, and the influence of the suc-
FIG. 2. FTIR spectrum of pentyl oleate succinic anhydride.
Wavenumber (cm
–1
)

cinic moiety. With regard to the stereoselectivity, previous
studies (7,15,16) have indicated that the mechanism of the-
ene reaction for a cis alkene (oleic acid) with a maleic anhy-
dride leads to 100% trans adducts. This change in configura-
tion and the presence of the ester and succinic moieties influ-
ences the intermolecular arrangement and consequently the
physical properties of vegetable ASA compounds.
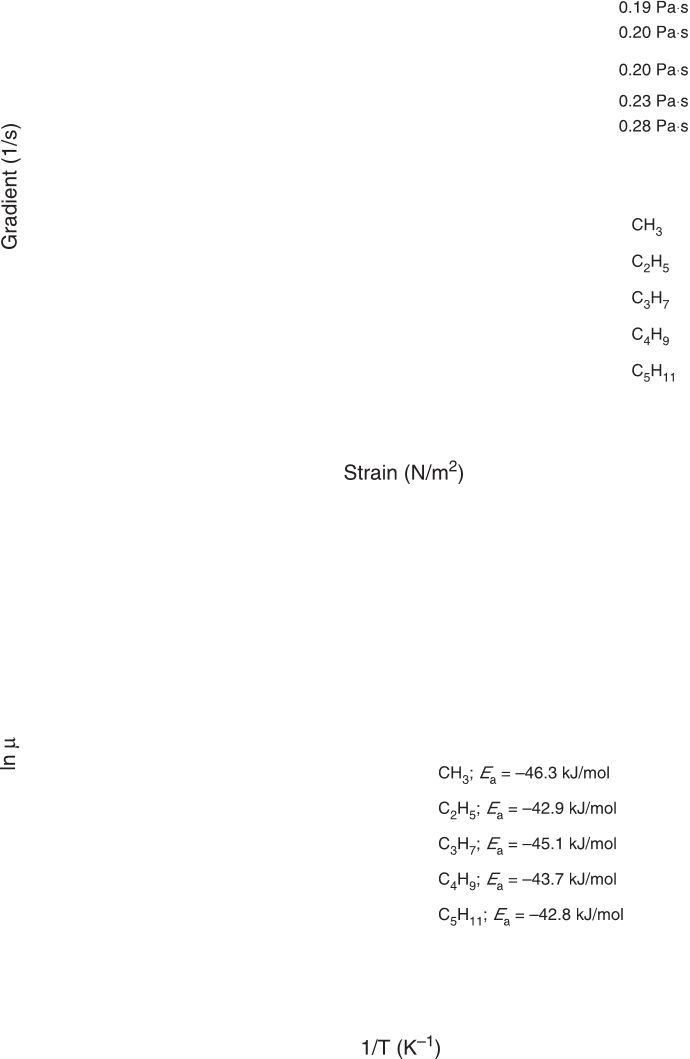
Viscosity. The rheological behavior of ASA was studied at
20°C (Fig. 3). The gradient was an increasing linear function
of the strain. The viscosity, i.e., the slope of the preceding
FIG. 3. Rheological behavior of
ASA. (A) Gradient vs. strain applied. (B) Dynamic viscosity vs. temperature.
Methyl oleate succinic anhydride (CH
3
), ethyl oleate succinic anhydride (C
2
H
5
), propyl oleate succinic an-
hydride (C
3
H
7
), butyl oleate succinic anhydride (C
4
H
9
), pentyl oleate succinic anhydride (C
5
H
11
). For other
abbreviation see Figure 1.
1/T (K
–1
)
Gradient (1/s)
ln µ
Strain (N/m
2
)
0.19 Pa⋅s
0.20 Pa⋅s
0.20 Pa⋅s
0.23 Pa⋅s
0.28 Pa⋅s
CH
3
C
2
H
5
C
3
H
7
C
4
H
9
C
5
H
11
CH
3
;
E
a
= –46.3 kJ/mol
C
2
H
5
;
E
a
= –42.9 kJ/mol
C
3
H
7
;
E
a
= –45.1 kJ/mol
C
4
H
9
;
E
a
= –43.7 kJ/mol
C
5
H
11
;
E
a
= –42.8 kJ/mol