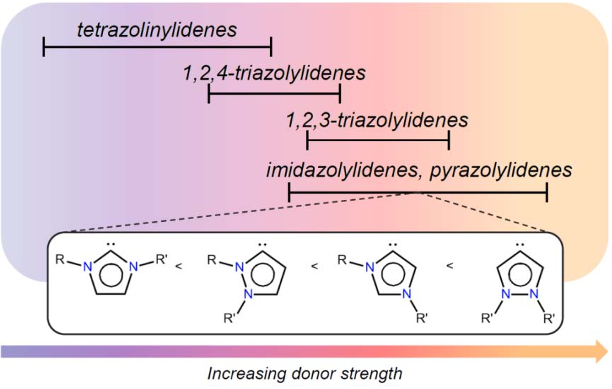
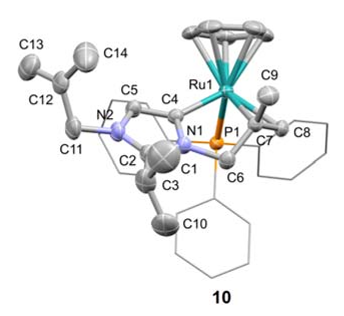
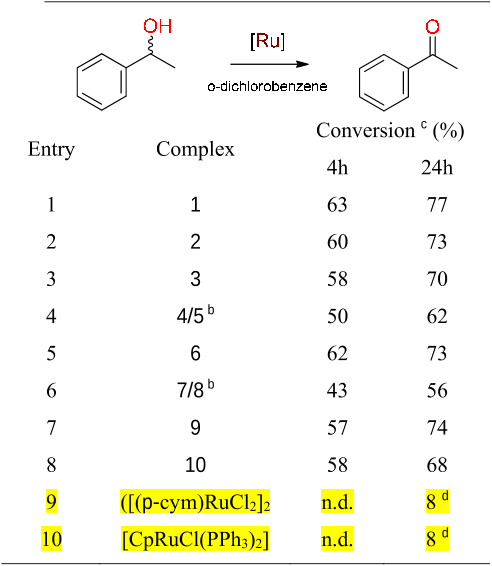
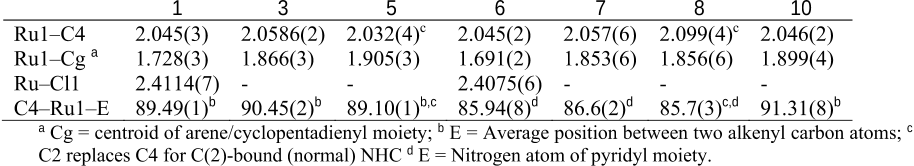Abstract: Analogues of [Ru(bpy)3]2+ were prepared in which one pyridine ligand site is substituted by a N-heterocyclic carbene (NHC) ligand, that is, either by an imidazolylidene with a variable wingtip group R (R = Me, 3a; R = Et, 3b; R = iPr, 3c), or by a benzimidazolylidene (Me wingtip group, 3d), or by a 1,2,3-triazolylidene (Me wingtip group, 3e). All complexes were characterized spectroscopically, photophysically, and electrochemically. An increase of the size of the wingtip groups from Me to Et or iPr groups distorts the octahedral geometry (NMR spectroscopy) and curtails the reversibility of the ruthenium oxidation. NHC ligands with methyl wingtip groups display reversible ruthenium oxidation at a potential that reflects the donor properties of the NHC ligand (triazolylidene > imidazolylidene > benzimidazolylidene). The most attractive properties were measured for the triazolylidene ruthenium complex 3e, featuring the smallest gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupi...