1
TCR affinity controls the dynamics but not the functional specification
1
of the Th1 response to mycobacteria
2
Nayan D Bhattacharyya
1,2
, Claudio Counoupas
3,2
, Lina Daniel
1,2
, Guoliang Zhang
4,1,2
, Stuart J
3
Cook
5
, Taylor A Cootes
1,2
, Sebastian A Stifter
1,2
, David G Bowen
7,8
, James A Triccas
3,2,6
, Patrick
4
Bertolino
7,8
, Warwick J Britton
2
& Carl G Feng
1,2,6*
5
6
Affiliations:
7
1
Immunology and Host Defense Group, Department of Infectious Diseases and Immunology,
8
School of Medical Sciences, Faculty of Medicine & Health, The University of Sydney, NSW,
9
2006, Australia.
10
2
Tuberculosis Research Program, Centenary Institute, Royal Prince Alfred Hospital,
11
Camperdown, NSW, 2050, Australia.
12
3
Microbial Pathogenesis and Immunity Group, Department of Infectious Diseases and
13
Immunology, School of Medical Sciences, Faculty of Medicine & Health, University of Sydney,
14
NSW, 2006, Australia.
15
4
National Clinical Research Center for Infectious Diseases, Guangdong Key Laboratory of
16
Emerging Infectious Diseases, Shenzhen Third People’s Hospital, Southern
17
University of Science and Technology, Shenzhen, China.
18
5
Immune Imaging Program, Centenary Institute, Royal Prince Alfred Hospital, Camperdown,
19
NSW, 2050, Australia.
20
6
Marie Bashir Institute for Infectious Diseases and Biosecurity, The University of Sydney,
21
Sydney, NSW, 2006, Australia.
22
7
Liver Immunology Program, Centenary Institute, Royal Prince Alfred Hospital, Camperdown,
23
NSW, 2050, Australia.
24
8
AW Morrow Gastroenterology and Liver Centre, Royal Prince Alfred Hospital, Camperdown,
25
NSW, 2050, Australia.
26
27
* Corresponding author: Carl G Feng: carl.feng@sydney.edu.au
28
29
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted October 26, 2020. ; https://doi.org/10.1101/2020.10.25.353763doi: bioRxiv preprint

2
Abstract:
30
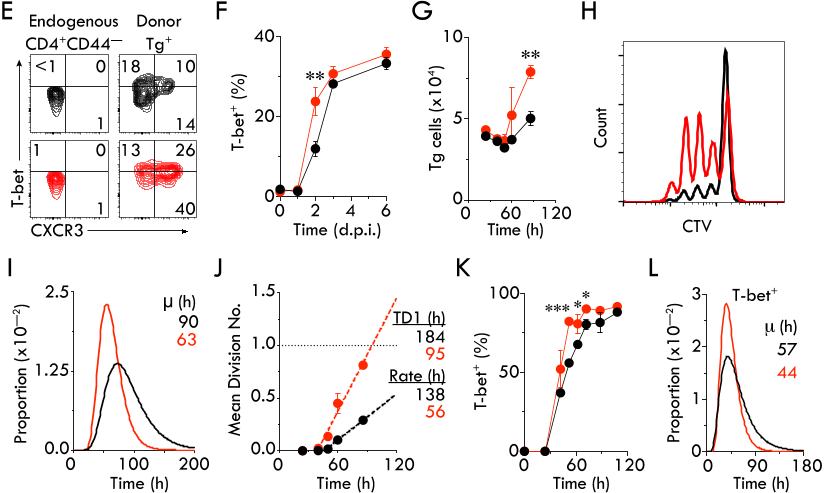
The quality of T cell responses depends on the lymphocytes’ ability to undergo clonal expansion,
31
acquire effector functions and traffic to the site of infection. Although TCR signal strength is
32
thought to dominantly shape the T cell response, by using TCR transgenic CD4
+
T cells with
33
different pMHC binding affinity, we reveal that TCR affinity does not control Th1 effector
34
function acquisition nor the functional output of individual effectors following mycobacterial
35
infection. Rather, TCR affinity calibrates the rate of cell division to synchronize the distinct
36
processes of T cell proliferation, differentiation and trafficking. By timing cell division-dependent
37
IL-12R expression, TCR affinity controls when T cells become receptive to Th1-imprinting IL-12
38
signals, determining the emergence and magnitude of the Th1 effector pool. These findings reveal
39
a distinct yet cooperative role for IL-12 and TCR signalling in Th1 differentiation and suggests
40
that the temporal activation of clones with different TCR affinity is a major strategy to coordinate
41
immune surveillance against persistent pathogens.
42
43
Keywords:
44
TCR affinity, TCR signal, Th1 response, cell division, IL-12, mycobacteria.
45
46
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted October 26, 2020. ; https://doi.org/10.1101/2020.10.25.353763doi: bioRxiv preprint

3
Introduction:
47
The successful containment of invading pathogens requires the rapid generation of large numbers
48
of antigen-specific T cells with the correct effector function. This involves the activation of distinct
49
programs, including T cell proliferation, differentiation, and migration. Failure in activating or
50
regulating these programs results in impaired host defense. The majority of studies have focused
51
on one or a limited number of CD4
+
T cell programs, such as, the magnitude of population
52
expansion or expression of master regulators of transcription. There is little information available
53
as to whether these processes, which operate at different biological scales spanning from the
54
molecule to the tissue, are individually or cooperatively regulated in vivo. Indeed, in vitro studies
55
have already suggested a link between cell division and differentiation, demonstrating that cell
56
division progression is associated with increased expression of signature Th cytokines (1-3).
57
The pool of naive T cells in vivo is diverse and contains clones that express distinct TCRs
58
recognizing different peptide:MHC (pMHC) complexes. It is estimated that there are anywhere
59
between twenty and one thousand naive T cells that possess the same pMHC specificity (4, 5),
60
each with different binding affinities. The strength of TCR signals, regulated by the TCRs affinity,
61
the density of pMHC and co-stimulatory molecules on antigen presenting cells (APCs), regulates
62
downstream T cell activation and function (6, 7). While high affinity TCR signals in cytotoxic
63
CD8
+
T lymphocytes accelerate cell division and prolong population expansion (8, 9), it delays
64
their migration from secondary lymphoid organs (SLOs) (9, 10) resulting in impaired pathogen
65
control (10). Similarly, strong TCR signals enhance the expansion of CD4
+
T cell populations (11-
66
13).
67
Defining the role of TCR signaling strength in the CD4
+
lymphocyte response is
68
challenging because of the functional heterogeneity in helper T cell populations. The effector
69
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted October 26, 2020. ; https://doi.org/10.1101/2020.10.25.353763doi: bioRxiv preprint

4
function of Th populations is instructed by signals from the TCR as well as from pathogen-
70
conditioned accessory cells and APCs. Historically, investigations into Th cell differentiation have
71
focused on “qualitative” T cell-extrinsic cytokine signals (7, 14). In the case of Th1 differentiation,
72
the innate cytokine IL-12 promotes the generation of interferon-γ (IFN-γ)-producing effectors (15,
73
16) and host survival following infection with intracellular pathogens (17). Recent studies have
74
suggested a role for “quantitative” differences in TCR signal strength in regulating CD4
+
T cell
75
differentiation (12, 18-20). Potent TCR signaling is associated with the generation of Th1 (18, 19)
76
or Tfh cells (11, 20, 21). Mechanisms proposed to mediate strong TCR signal-driven Th lineage
77
commitment vary depending on experimental settings. For example, IL-2 (12, 13, 19) and IL-12
78
receptor signaling (18) have each been suggested to contribute to the generation of Th1 populations
79
following potent TCR stimulation. The model-dependent function of strong TCR signaling
80
suggests a potential interplay between quantitative TCR and qualitative environmental signals in
81
instructing Th differentiation. Currently, the relative role of TCR and innate cytokine signals in
82
lineage commitment and the mechanisms integrating these signals is unknown.
83
In this study, we developed a T cell adoptive transfer model using CD4
+
T cells from two
84
TCR transgenic (Tg) mouse lines that recognize the same epitope of the Mycobacterium
85
tuberculosis protein Early Secretory Antigenic Target 6 (ESAT-6, E6), with different binding
86
affinities. Following T cell transfer, WT or IL-12-deficient recipient mice were infected with a
87
recombinant Mycobacterium bovis Bacillus Calmette-Guérin-expressing E6 (BCG-E6). By
88
tracking transgenic CD4
+
T cells across multiple time-points and in different tissues following
89
intravenous (i.v.) BCG infection, we reveal that by adjusting the rate of cell division, a major
90
function of TCR affinity is to determine the speed and magnitude of the CD4
+
T cell response.
91
Moreover, TCR affinity plays a minimal role in specifying T helper cell effector function.
92
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted October 26, 2020. ; https://doi.org/10.1101/2020.10.25.353763doi: bioRxiv preprint

5
However, by regulating cell division-dependent IL-12Rb2 expression, TCR affinity controls when
93
T cells become receptive to IL-12 and acquire Th1 effector function. Since high affinity CD4
+
T
94
cells also migrate to infected non-lymphoid tissues faster than their low affinity counterparts, our
95
findings show that TCR affinity coordinates multiple programs to determine the overall potency
96
of the Th cell response to infection. They also suggest that the temporal activation of distinct T
97
cell clones is a mechanism controlling the initiation and maintenance of Th1 immunity against
98
persistent infection.
99
100
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted October 26, 2020. ; https://doi.org/10.1101/2020.10.25.353763doi: bioRxiv preprint