HAL Id: hal-03177581
https://hal.archives-ouvertes.fr/hal-03177581
Submitted on 23 Mar 2021
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-
entic research documents, whether they are pub-
lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diusion de documents
scientiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
The Pt-Catalyzed Ethylene Hydroamination by Aniline:
A Computational Investigation of the Catalytic Cycle
Pavel Dub, Rinaldo Poli
To cite this version:
Pavel Dub, Rinaldo Poli. The Pt-Catalyzed Ethylene Hydroamination by Aniline: A Computational
Investigation of the Catalytic Cycle. Journal of the American Chemical Society, American Chemical
Society, 2010, 132 (39), pp.13799-13812. �10.1021/ja1051654�. �hal-03177581�

1
The Pt-catalyzed ethylene hydroamination by aniline: a Computational
Investigation of the Catalytic Cycle
Pavel A. Dub
a
and Rinaldo Poli*
,a,b
a
CNRS; LCC (Laboratoire de Chimie de Coordination); Université de Toulouse; UPS, INP; F-
31077 Toulouse, France ; 205, route de Narbonne, F-31077 Toulouse, France; Fax: (+) 33-
561553003; E-mail: poli@lcc-toulouse.fr
b
Institut Universitaire de France, 103, bd Saint-Michel, 75005 Paris, France

2
Summary
A full QM DFT study without system simplification and with the inclusion of solvation effects in
aniline as solvent has addressed the addition of aniline to ethylene catalyzed by PtBr
2
/Br
-
. The
resting state of the catalytic cycle is the [PtBr
3
(C
2
H
4
)]
-
complex (II). A cycle involving aniline
activation by N-H oxidative addition was found energetically prohibitive. The operating cycle
involves ethylene activation followed by nucleophilic addition of aniline to the coordinated
ethylene, intramolecular transfer of the ammonium proton to the metal center to generate a 5-
coordinate (16- electron) Pt
IV
-H intermediate, and final reductive elimination of the PhNHEt
product. Several low energy ethylene complexes, namely trans- and cis-PtBr
2
(C
2
H
4
)(PhNH
2
)
(IV and V) and trans- and cis-PtBr
2
(C
2
H
4
)
2
(VII and VIII) are susceptible to aniline
nucleophilic addition to generate zwitterionic intermediates. However, only
[PtBr
3
CH
2
CH
2
NH
2
Ph]
-
(IX) derived from PhNH
2
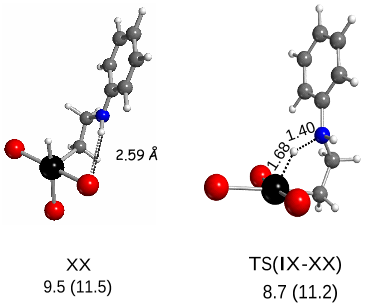
addition to II is the productive intermediate. It
easily transfers a proton to the Pt atom to yield [PtHBr
3
(CH
2
CH
2
NHPh)]
-
(XX), which leads to
rate-determining C-H reductive elimination through transition state TS(XX-L) with formation of
the -complex [PtBr
3
(k
2
:C,H-HCH
2
CH
2
NHPh)]
-
(L), from which the product can be liberated
via ligand substitution by a new C
2
H
4
molecule to regenerate II. Saturated (18-electron) Pt
IV
hydride complexes obtained by ligand addition or by chelation of the aminoalkyl ligand liberate
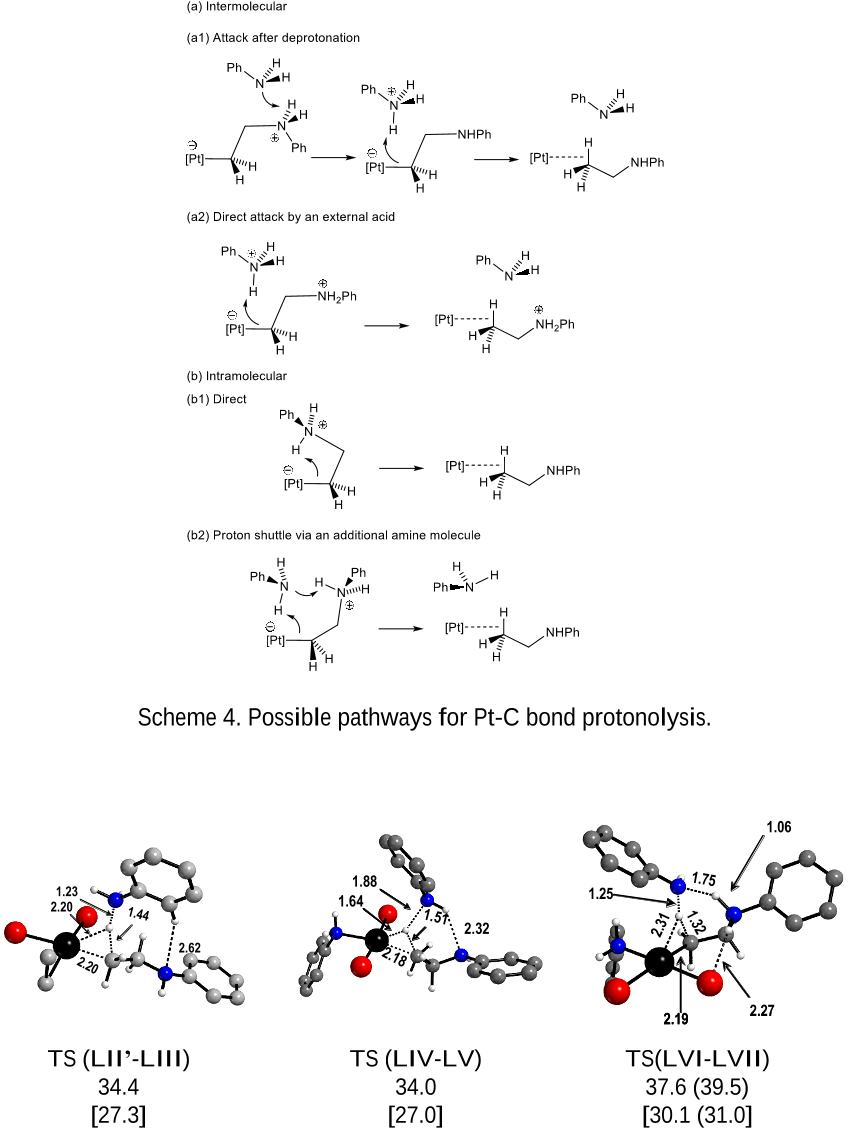
the product through higher energy pathways. Other pathways starting from the zwitterionic
intermediates were also explored (intermolecular N deprotonation followed by C protonation or
chelation to produce platina(II)azacyclobutane derivatives; intramolecular proton transfer from N
to C, either direct or assisted by an external aniline molecule) but all gave higher-energy
intermediates or led to the same rate determining TS(XX-L).
Keywords: platinum, homogeneous catalysis, hydroamination, non-activated olefins, DFT
calculations

3
Introduction
Hydroamination, the direct formation of a new C-N bond by addition of an N-H bond
across an unsaturated CC bond, currently attracts much interest in academia and industry.
1-3
The
intermolecular version of this process is still a great challenge, especially for non-activated
olefins. Seminal work by Coulson showed that ethylene could be hydroaminated by a few highly
basic secondary amines under forcing conditions with RhCl
3
(or IrCl
3
) as catalyst.
4, 5
More
recently, this system was found effective also for less basic amines such as aniline when
modified by the addition of n-Bu
4
PI/I
2
.
6
Other relevant results for the intermolecular
hydroamination of ethylene and other non activated olefins comprise the use of lanthanides,
7, 8
Fe,
9
Ru,
10-12
Rh,
13
Ag,
14
Au,
15-18
Pd,
19, 20
and notably Pt.
21-23
Investigations initiated in our team
by J.-J. Brunet have shown that PtBr
2
, in the presence of nBu
4
PX (X = halide) as activator, is one
of the most performing catalyst so far reported for the hydroamination of ethylene by weakly
basic amines such as aniline and 2-chloroaniline (highest activity for X = Br; TON > 150 after
10 h at 150°C with 0.3 mol % of Pt- precursor).
24-27
Without a clear mechanistic understanding,
however, it is difficult to imagine how to further improve the process efficiency for its potential
application in bulk chemical manufacture.
Two alternative mechanisms are discussed in the literature, one starting with amine
activation by N-H oxidative addition and the other one based on amine nucleophilic addition to a
coordinated olefin. The amine activation mechanism is mostly proposed for Rh- or Ir-based
catalytic systems,
28, 29
whereas the olefin activation mechanism seems adopted by catalysts based
on group 10
30
and 11 metals.
31
Senn and coworkers reported a computational study of the model
NH
3
addition to ethylene catalyzed by the [MCl(PH
3
)(C
2
H
4
)]
z+
complexes of Group 9 (z = 0) and
10 (z = 1) metals.
32
For the group 10 metals, for which only the olefin activation pathway has
been explored, they found that the NH
3
nucleophilic addition is thermodynamically and
kinetically favourable and that the cleavage step is rate-determining (barrier of 34.9 kcal mol
-1

4
for Pt). On the other hand, Tsipis and Kefalidis, using the “Pt
0
” model complex Pt(C
2
H
4
)(PH
3
),
explored only the amine activation pathway, finding the reaction to be limited by the product
reductive elimination step from the Pt
II
amido hydrido intermediate (barrier of 39.7 kcal mol
-1
).
33
Other computational studies (e.g. on gold catalysis for diene hydroamination
31
or palladium
catalysis for the intermolecular hydroamination of vinylarenes
34
and for the asymmetric
intramolecular hydroamination of aminoalkenes
35
) have also explored solely the olefin activation
mechanism. To the best of our knowledge, with the exception of the above mentioned study by
Tsipis and Kefalidis and a study on iridium reported only in a Ph.D. thesis,
36
studies of the N-H
activation pathway have only been reported for alkaline-earths,
37
early transition metals
38-41
and
the lanthanides.
42-48
On the basis of known chemical transformations for related systems and on conventional
wisdom, the Brunet Pt-based system was proposed to follow the ethylene activation pathway as
shown in Scheme 1.
26
However, whether the proton transfer from N to C from the zwitterionic
intermediate occurs directly or via a Pt-hydride intermediate remained open to debate. The
proton transfer process was considered as more facile from the anionic tribromo species
[PtBr
3
(CH
2
CH
2
NH
2
Ph)]
-
because of the anticipated increased basicity, a hypothesis consistent
with the observed activity enhancement when using a moderate excess amount of bromide
salts.
24
+CH
2
=CH
2
-Br
-
+ArNH
2
-Br
-
[PtBr
4
]
2-
+ArNH
2
[(ArNH
2
)PtBr
2
CH
2
CH
2
-NH
2
Ar]
+ArNH
2
ArNHCH
2
CH
3
H
+Br
-
-ArNH
2
[PtBr
2
] + 2 Br
-
[PtBr
3
]
-
+ Br
-
[PtBr
3
(C
2
H
4
)]
-
[PtBr
3
CH
2
CH
2
-NH
2
Ar]
-
[PtBr
3
CH
2
CH
2
-NHAr]
-
[PtBr
2
(ArNH
2
)(C
2
H
4
)]