




Did you find this useful? Give us your feedback

























85 citations
...This work was supported by the Human Resources Program in Energy Technology of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), which was granted financial resources from the Ministry of Trade, Industry & Energy, Republic of Korea (20194010201890)....
[...]
...117 D. P. Raphaël, The recycling of lithium-ion batteries A strategic pillar for the European Battery Alliance, Center for Energy, Raphael, 2020....
[...]
...Energy, 2019, 4, 253....
[...]
...Energy, 2013, 110, 252....
[...]
41 citations
3 citations
1 citations
...Because in previous work of this recycling project, H2SO4 provided higher leaching efficiency than HCl and HNO3 [73]....
[...]
...The former is the most effective reducing agent based on previous work of this project [73] while the latter is emerging as an new promising reducing agent in recent recycling research....
[...]
This degradation, along with the battery being completely exhausted are key to the formation of grain boundaries present in Fig. 17 (A) and (B).
Due to the extreme temperatures involved in pyrometallurgy, methods such as the Toxco and Recupyl are preferred to that created by Umicore.
The batteries are milled in lithium brine, in which the lithium dissolves with several salts forming lithium chloride (LiCl) (Saloojee and Lloyd 2015).
It was noted that the powdered samples, in which had not been ground into a fine powder prior to leaching, were slowly being disintegrated as the solution colour seemingly darkened in colour.
With acid leaching pivotal to the hydrometallurgical process, it highlights the importance of determining an efficient and effective leachant.
the powdered samples appear marginally darker than the panel samples, in part due to the acid covering a larger surface area of the lithium cobalt oxide solid.
In the current era, lithium-ion batteries are typically used in portable handheld electronic devices such as mobile phones and laptop PC’s.
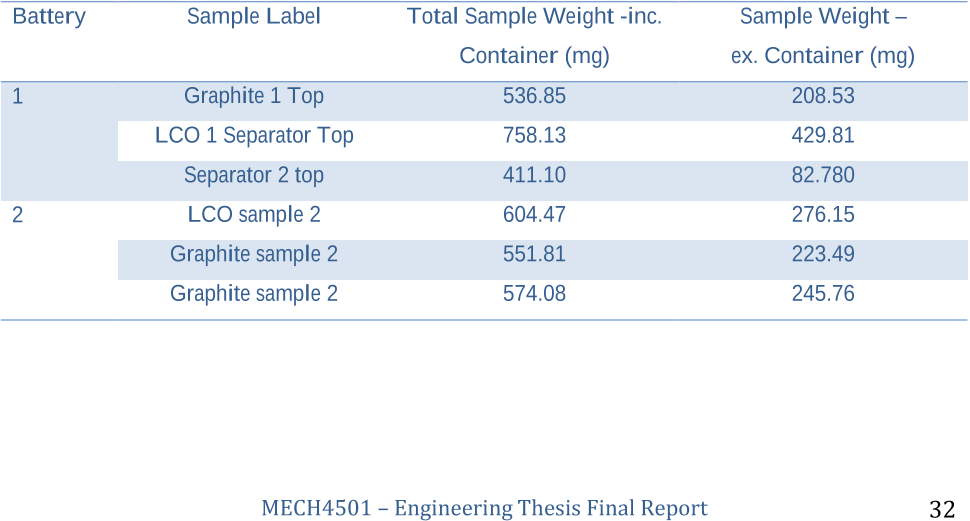
Initial ICP AnalysisSamples of lithium cobalt oxide and graphite were carefully removed from the aluminium and copper foils respectively.
As each battery originated from an iPhone, initially a 3.7 V LiCoO2 battery, the typical end-of-discharge range is from 2.8-3.0 V (Cadex 2018).
Using the recovered lithium cobalt oxide, from the original iPhone battery, two cathode pastes were produced one ratio with additional carbon (Super P) and one without.
Due to the tested battery voltages being below the acceptable discharge range it is difficult to determine exactly what component the lithium resides in.
The remaining material, after ICP testing, was used to prepare 4 vials each containing 500 mg of the recovered LCO material along with 4 vials containing a single panel of the top separator.
This shows that the non-super P batteries do not contain enough carbon, which acts as a conductor for the electric energy in the cell.
The mapping confirms the large remaining grains to be the cobalt oxide compounds with the majority of the dark wash between them as the sulphuric acid.