UCSF Chimera—A Visualization System for Exploratory
Research and Analysis
ERIC F. PETTERSEN, THOMAS D. GODDARD, CONRAD C. HUANG, GREGORY S. COUCH,
DANIEL M. GREENBLATT, ELAINE C. MENG, THOMAS E. FERRIN
Computer Graphics Laboratory, Department of Pharmaceutical Chemistry, University of
California, 600 16th Street, San Francisco, California 94143-2240
Received 24 February 2004; Accepted 6 May 2004
DOI 10.1002/jcc.20084
Published online in Wiley InterScience (www.interscience.wiley.com).
Abstract: The design, implementation, and capabilities of an extensible visualization system, UCSF Chimera, are
discussed. Chimera is segmented into a core that provides basic services and visualization, and extensions that provide
most higher level functionality. This architecture ensures that the extension mechanism satisfies the demands of outside
developers who wish to incorporate new features. Two unusual extensions are presented: Multiscale, which adds the
ability to visualize large-scale molecular assemblies such as viral coats, and Collaboratory, which allows researchers to
share a Chimera session interactively despite being at separate locales. Other extensions include Multalign Viewer, for
showing multiple sequence alignments and associated structures; ViewDock, for screening docked ligand orientations;
Movie, for replaying molecular dynamics trajectories; and Volume Viewer, for display and analysis of volumetric data.
A discussion of the usage of Chimera in real-world situations is given, along with anticipated future directions. Chimera
includes full user documentation, is free to academic and nonprofit users, and is available for Microsoft Windows,
Linux, Apple Mac OS X, SGI IRIX, and HP Tru64 Unix from http://www.cgl.ucsf.edu/chimera/.
© 2004 Wiley Periodicals, Inc. J Comput Chem 25: 1605–1612, 2004
Key words: molecular graphics; extensibility; visualization; multiscale modeling; sequence alignment
Introduction
Since its inception, the UCSF Computer Graphics Laboratory has
worked on molecular visualization systems to meet the needs of
researchers in the field, beginning with MMS/MIDS
1
in 1976 to
our current offering, UCSF Chimera
2
(henceforth “Chimera”).
Chimera’s immediate predecessor, the Midas/MidasPlus system
3
(henceforth “Midas”), provided us with the insight that extensibil-
ity should be considered critically important in the design of a
visualization system.
Midas was a highly successful molecular graphics system.
However, it was relatively difficult for users to add new function-
ality. The first extension mechanism introduced into Midas was the
ability to send an annotated Protein Data Bank (PDB)
4
file repre
-
senting the currently displayed scene to an external program.
5
This
was motivated by our desire to interface to rendering programs
such as RASTER3D.
6
Once this extension mechanism was avail
-
able, it became relatively easy to develop rendering “back ends” of
our own, and soon thereafter we developed both a fast space-filling
renderer with shadows, Conic,
7
and a Jane Richardson-style
8
rib
-
bon-depiction program with many capabilities, Ribbonjr. Further-
more, outside developers exploited the mechanism in ways that we
had not anticipated. Thomas Hynes (then with Genentech) wrote
Neon, a program to allow Midas to depict shadowed ball-and-stick
scenes. Neon acted as a filter between Midas and Conic, taking the
PDB file output by Midas and producing another PDB file with an
order of magnitude more atoms. Neon output contained larger-
radius atoms for balls and series of many closely spaced, smaller-
radius atoms to simulate sticks. To our surprise, Conic was able to
process the Neon file quickly enough (seconds to minutes on
computers of the day) to be usable. This whole Neon concept was
something that would never have occurred to us, and opened our
eyes to the power of an effective extension mechanism.
A second extension mechanism was subsequently created to
allow Midas to communicate on an ongoing basis with external
programs. The Midas user could send commands to an external
program and the external program could issue Midas commands to
cause changes in the Midas session. Although this mechanism was
also quite successful, it was ultimately constrained to the available
Midas command set. The restrictions imposed by the original
Correspondence to: T.E. Ferrin; e-mail: tef@cgl.ucsf.edu
Contract/grant sponsor: NIH; contract/grant number: P41-RR01081
© 2004 Wiley Periodicals, Inc.

Midas design were proving problematic, and we were motivated to
create a new system with greater extensibility.
Chimera was designed with extensibility as a primary goal. We
also wanted Chimera to be portable to a wide variety of platforms,
and to include state-of-the-art graphics capabilities such as trans-
parency and interactive ball-and-stick, space-filling, ribbon, and
solid surface representations. Another design goal was to make
Chimera accessible to users at all skill levels by providing both a
graphical menu/window interface and a command-line interface.
Chimera Architecture
Chimera’s primary programming language is Python.
9
Impor
-
tantly, Python is an interpreted, object-oriented programming lan-
guage that is also easy to learn and very readable. Because Python
is interpreted, it is good for rapid development and debugging.
Readability is important for a team development project like
Chimera, and an easy-to-learn language enables others to develop
extensions without undue effort. Chimera includes the Python-
standard IDLE interactive development environment
10
to help
diagnose problems during extension development.
Chimera is divided into a core and extensions. The core pro-
vides basic services and molecular graphics capabilities. All higher
level functionality is provided through extensions. This design,
with the bulk of Chimera functions provided by extensions, en-
sures that the extension mechanism is robust enough to handle the
needs of outside researchers wanting to extend Chimera in novel
ways. Extensions can be integrated into the Chimera menu system,
and can present a separate graphical user interface as needed using
the Tkinter,
11
Tix,
12
and/or Pmw
13
toolkits.
The Chimera core consists of a C⫹⫹ layer that handles time-
critical operations (e.g., graphics rendering) and a Python layer
that handles all other functions. All significant C⫹⫹ data and
functions are made accessible to the Python layer. Core capabili-
ties include molecular file input/output, molecular surface gener-
ation using the MSMS algorithm,
14
and aspects of graphical dis
-
play such as wire-frame, ball-and-stick, ribbon, and sphere
representations, transparency control, near and far clipping planes,
and lenses (screen areas with different display attributes; see
Fig. 1).
Another core service is maintenance and display of the current
selection. Users may select parts of structures by picking with the
mouse, by making menu choices (e.g., aromatic rings), or via
certain extension actions. The selected structure areas are high-
lighted with a particular color or a colored outline. Extensions can
query for the contents of the selection. Many menu actions (such
as coloring or setting the display style) work on the current
selection.
Figure 1. A single frame of a molecular dynamics trajectory of a
buckytube in water, shown with the Movie extension. A “lens” has
been placed over the center of the tube to strip away the surface and
reveal the hydrogen-bonded chain of waters passing through the tube.
The trajectory was computed with polarizable molecular dynamics
using the Amber 8
26
Sander module (J. Caldwell, UCSF, unpublished
data).
Figure 2. Bluetongue virus core particle (PDB identifier 2btv
16
) with
double-stranded RNA attached to the surface (1h1k
17
) (top). Trimers
in the outer protein layer that are equivalent under the icosahedral
symmetry are given the same color. The free end of the RNA attaches
to other viral particles in the crystal. A closeup of the inner layer
(bottom) shows ball-and-stick and ribbon models and a surface at
higher resolution.
1606 Pettersen et al. • Vol. 25, No. 13 • Journal of Computational Chemistry

The core also maintains a trigger mechanism wherein changes
to core data structures or state are reported to extensions that have
registered callbacks with the corresponding trigger. For example,
there is a “selection changed” trigger that fires whenever the
current selection changes.
Extensions are written either entirely in Python or in a combi-
nation of Python and C/C⫹⫹ (the latter using a shared library
loaded at runtime). Extensions can be placed in the Chimera
installation directory (which would make the extension available
to all users) or in the user’s own file area. Extensions are loaded on
demand, typically when the user accesses a menu entry that starts
the extension. The class structure of molecular data and other
extension programming information can be found at http://www.
cgl.ucsf.edu/chimera/docs/ProgrammersGuide/Examples/.
We demonstrate Chimera’s extensibility by presenting several
extensions. The Multiscale and Collaboratory extensions are quite
unique, and demonstrate the wide spectrum of abilities that can be
generated. The others provide insight into the use of core facilities
by extensions and the integration of extensions with the Chimera
environment.
Multiscale
The Multiscale extension adds capabilities for interactively explor-
ing large molecular assemblies. We have focused on viral struc-
tures, condensed chromosomes, and ribosomes; additional exam-
ples include cytoskeletal fibers and motors, flagellar structures, and
chaperonins. The Multiscale extension displays structures from the
PDB
4
and generates their multimeric forms by using transforma
-
tion matrices to position the subunits. Multiscale can also be used
with large assemblies where there are no repeated subunits. PDB
chains can be displayed as low-resolution surfaces, or in any of the
standard molecular representations available in Chimera. Multi-
scale permits biologically meaningful levels of quaternary struc-
ture to be defined. The abilities to build multimeric forms, display
low-resolution representations, and define levels of structure are
important for interactively exploring large complexes.
Multiscale uses Chimera’s core molecular display abilities, data
structures, file reading, and selection management, and the Volume
Viewer extension for surface calculation and rendering. This
ready-to-use infrastructure allowed us to focus on the new capa-
bilities needed for displaying complexes.
Most of the available atomic-resolution viral structures are
icosahedral particles with 60-fold symmetry. PDB files provide
atomic coordinates for only one subunit. To build a multimeric
model, subunits are positioned using rotation/translation matrices
read from the PDB file header. PDB “REMARK 350” records give
matrices that can be used to generate the biological oligomeriza-
tion state. Crystallographic (SMTRY records) and noncrystallo-
graphic symmetry (MTRIX records) matrices or matrices inferred
from the space group (CRYST1 record) of crystal structures can
also be used. Multiscale’s visualization capabilities have revealed
shortcomings in the matrix information for many large-scale struc-
ture entries that otherwise would have been difficult to detect. We
are working with the PDB to find and correct these entries.
For efficiency, the Multiscale extension only loads atomic
coordinates for subunits when they are needed. When a model is
first displayed, only a low-resolution surface representation is
shown, so no additional copies of the coordinates need to be
loaded. Chimera’s atomic and residue-level display styles are also
available, but are typically used for only a small number of
subunits so that the amount of detail depicted does not overwhelm
the user. The Multiscale extension does not use the high-resolution
molecular surfaces that are a core feature of Chimera. Such sur-
faces would render too slowly for a large multimer and provide too
high a level of detail to best illustrate the organization of subunits.
The Multiscale extension was originally written entirely in
Python. To speed up the surface calculation, certain critical rou-
tines were rewritten in C⫹⫹. Converting these routines from
interpreted Python to compiled C⫹⫹ made them run about 50
times faster. Translation is generally straightforward because Py-
thon objects such as molecules, atoms, and lists have equivalent
C⫹⫹ objects. Rendering the surface with OpenGL,
15
another
time-critical step, uses the C⫹⫹ module in the Volume Viewer
extension.
Subunits can be selected with the mouse. To simplify selecting
larger pieces of a structure, new structure levels can be defined
hierarchically. For example, the bluetongue virus core particle
16
has two protein layers, the outer layer being composed of 260
trimers (Fig. 2). For this structure, it is useful to define inner and
outer layers and trimers as structural levels. After an individual
outer layer monomer is selected, the selection can then be pro-
moted to the containing trimer, and subsequently promoted to the
whole layer of trimers. The whole outer layer can then be hidden
if the object of interest is the inner protein layer. Besides being
promoted, a selection can be extended to all identical copies of the
currently selected subunits. It is also possible to select just the
subunits for which atomic information has been loaded.
Structure levels can be specified in a Python script. Structural
hierarchies are sometimes described in text in the headers of PDB
files. The mmCIF file format available from the PDB
4
has a limited
ability to describe such higher levels of structure in a computer-
readable form, but few submitted data sets provide this informa-
tion.
For multimeric structures, investigations are often facilitated by
the presence of more than one copy of the asymmetric unit. The
bluetongue virus structure
16,17
illustrates how working with the
full viral shell can aid analysis. The crystallographic data used to
determine the capsid structure revealed viral double-stranded RNA
stuck to the outside of the particles
17
(Fig. 2). To investigate the
specific atomic contacts between the RNA and virus, it is helpful
to locate the several subunits of the icosahedral particle adjacent to
the RNA by inspecting the full particle. These can then be exam-
ined using an all-atom display to determine the contacts account-
ing for the stickiness of the capsid.
The Multiscale extension is intended for problems where both
large-scale and atomic-scale details are relevant. The tools needed
to explore models with many levels of structure and large numbers
of atoms are necessarily complex; the Chimera Multiscale exten-
sion has addressed only the most immediate needs. We anticipate
increasing its capabilities significantly in future releases.
Multalign Viewer
The Multalign Viewer extension allows Chimera to display se-
quence alignments together with associated structures (Fig. 3).
UCSF Chimera 1607

Multalign Viewer can read and write sequence alignments in a
wide variety of popular formats (currently Clustal
18
ALN,
“aligned” FASTA, GCG MSF, GCG RSF, “aligned” NBRF/PIR,
and Stockholm).
Multalign Viewer facilitates analysis of alignments in the con-
text of structural information and vice versa. First, structures in
Chimera must be associated with their corresponding sequences in
an alignment. When Multalign Viewer opens an alignment, it
examines the structures currently open in Chimera and checks each
chain for high sequence identity with an alignment sequence.
Chains with high identity are associated with the best-matching
sequence (however, only one chain per structure is associated with
a sequence). Multalign Viewer registers with the Chimera core’s
“model opened” trigger so that as new structures are opened, they
will also be examined and associated if appropriate. Conversely, if
the alignment sequence names are recognizable as including
SCOP
19,20
or PDB
4
identifiers using a few simple criteria, the
researcher can use a Multalign Viewer menu item or preference
setting to load all of the corresponding structures into Chimera.
This was easy to implement, because the Chimera core offers
functions for opening PDB/SCOP files based on their identifiers,
and will retrieve them via the World Wide Web or local disk as
appropriate. If Multalign Viewer fails to make an appropriate
automatic association between a sequence and structure, it can be
manually directed to make the association. Associations are indi-
cated by showing the color of the associated model behind the
name of the sequence (Fig. 3).
Once associations have been set up, many useful features
become active. Positioning the mouse over a sequence character
shows the number of the corresponding residue (in the structure) in
a status area. Making selections on the structures highlights the
corresponding regions of the sequence alignment. Dragging boxes
on the sequence alignment selects and highlights the correspond-
ing structure regions. Structure regions can be selected based on
conservation in the alignment, greatly facilitating coloring by
conservation level or showing only conserved side chains. Sec-
ondary structure elements can be depicted on the alignment with
colored boxes. Clicking on residues in the sequence makes the
Chimera window zoom in on the corresponding structure residues.
Structures can be superimposed using the sequence alignment,
optionally using only highly conserved residues, and also option-
ally, iteratively refining the fit by pruning poorly superimposed
residues.
An alignment can be searched with a literal string or a PROS-
ITE
21
pattern. Matches are highlighted on the alignment, and can
also be highlighted on associated structures.
Other extensions can call Multalign Viewer to show align-
ments. For example, the SSD (Structure Superposition Database)
22
extension uses Multalign Viewer to show sequence alignments
corresponding to structural alignments of interest.
Multalign Viewer is under active development. Important
short-term goals are to provide more sophisticated editing facilities
and to display and interact with phylogenetic information.
ViewDock
The ViewDock extension facilitates interactive screening of ligand
orientations from DOCK.
23,24
DOCK calculates possible binding
orientations given the structures of ligand and receptor molecules;
often, a large database of compounds is searched against a target
protein, where each compound is treated as a ligand and the target
is treated as the receptor. Simple scoring methods are used to
identify the most favorable binding modes of a given molecule and
then to rank the molecules. The output consists of a large number
of candidate ligands in the binding orientations considered most
favorable by DOCK.
Figure 3. Three structures in Chimera associated with sequences in an
alignment shown by Multalign Viewer. The sequences with color
swatches behind their names are associated with the pectate lyase struc-
tures 1jta,
45
1bn8,
46
and 2pec
47
(shown in yellow, magenta, and cyan,
respectively). The structures were superimposed using the sequence align-
ment; the fits were refined by iteratively removing bad residue pairings.
The sequences are colored by secondary structure (strand and helix
regions are pink and gold, respectively) and selected structure regions are
green (indicated on the structures with a green outline). Chimera’s zone
selection method was used to select all residues within 3.6 Å of the
active-site metal ion in one of the structures.
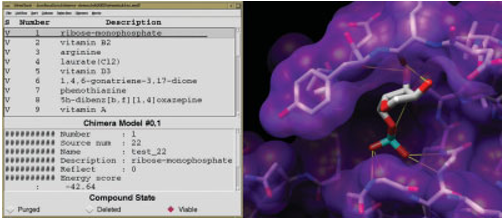
Figure 4. The ViewDock interface lists docked molecules; clicking
on a line displays just the corresponding molecule and shows its
information in the lower part of the panel. Ribose monophosphate is
shown docked to H-Ras (121p
48
). Carbon atoms are light gray, oxygen
atoms are red, nitrogen atoms are blue, and phosphorus atoms are
cyan. Hydrogens are not shown. Potential hydrogen bonds are indi-
cated with yellow lines.
1608 Pettersen et al. • Vol. 25, No. 13 • Journal of Computational Chemistry

ViewDock reads the DOCK output and provides a convenient
interface for filtering results in the context of the target structure.
When a line in the list of compounds is clicked, just the corre-
sponding molecule is shown in the putative binding site, and its
information is shown in the lower part of the panel (Fig. 4). The
information may include compound name, description, and various
scores and score components. Any of the descriptors can be shown
in the list and/or used to sort it. It is also possible to view more than
one docked molecule at a time.
Compounds can be deleted from the list if visual inspection
reveals them to be unsuitable. Compounds can also be screened by
the number of hydrogen bonds formed with the target structure and
by whether or not the hydrogen bonds involve specified groups in
the site. Hydrogen-bond detection uses a set of detailed distance
and angle criteria from a published small-molecule crystal sur-
vey
25
and is an extension-provided capability of Chimera.
Movie
The Movie extension allows Chimera to show molecular dynamics
trajectories. The trajectories may be played forward or backward,
either a single frame at a time or continuously. All generic Chi-
mera capabilities such as coloring, hydrogen-bond detection,
lenses (Fig. 1), and saving PDB files are available for use with the
trajectory. Movie explicitly supports execution of a script (Python
or Chimera commands) at each frame. This makes it easy (for
example) to save images for later assembly into a QuickTime or
MPEG video.
Movie currently supports all versions of AMBER
26
trajectory
files, and support for GROMOS
27
and NAMD
28
formats is in
progress.
Volume Viewer
The Volume Viewer extension displays three-dimensional (3D)
grid data such as density maps from electron microscope recon-
structions or X-ray crystallography, calculated electrostatic poten-
tial, and solvent occupancy from molecular dynamics simulations.
It reads several file formats (CCP4
29
or MRC, BRIX or DSN6,
30
CNS
31
or XPLOR,
32
SPIDER,
33
DelPhi
34
or GRASP
35
potential
maps, PRIISM,
36
NetCDF,
37,38
and DOCK
23
scoring grids), dis
-
plays isosurfaces, meshes, and translucent solids, and allows in-
teractive adjustment of thresholds, transparency, and brightness.
Volume data is often displayed with related molecular models.
The display is automatically updated when settings are
changed. For example, dragging a threshold indicator shown on a
histogram of data values updates the displayed surface or mesh.
For large data sets, subsampling can be used to improve the
response time when the display is rotated or a threshold is changed.
Subsampling with step size 2 renders the data after omitting every
other data plane along each axis. Data sets of 256 by 256 by 256
values can be displayed with a new threshold once a second or
rotated at 10 frames per second on generic desktop PC hardware
equipped with a mid-range graphics adapter. A subregion of the
data can be chosen by dragging a box with the mouse and then
shown instead of the whole data set. Subregions can be named so
that it is easy to return to them in later sessions.
The Volume Path Tracer extension allows marker placement
and path tracing in grid data. Markers are placed by mouse-click;
the marker is positioned on the closest visible data maximum along
the line of sight under the cursor. Markers can be moved after they
are placed. Consecutively placed markers can be linked with
segments to trace a path. Additional connections can be added with
the mouse to build simple structural models. Volume Path Tracer
was developed to trace protein backbones in intermediate-resolu-
tion (5–8 Å) density maps from electron cryo-microscopy (Fig. 5)
and fluorescently labeled chromosomes in 3D multiwavelength
light microscopy data (Fig. 6).
Markers and associated connecting segments are implemented
using the same mechanism as atoms and bonds. Thus, display
styles and colors can be changed in the same way as for atoms and
bonds, distances between pairs of markers and between markers
and molecular structures can be measured, and traced structures
can be aligned using Chimera’s molecule manipulation capabili-
ties. Traced paths can be displayed as smoothly interpolated curves
(Fig. 6). Markers and connecting segments can be saved in an
XML
39
file for analysis by other software.
Collaboratory
Chimera’s Collaboratory extension enables researchers at geo-
graphically distant locations to share a molecular modeling session
in real time. By default, all users connected to the same session
have equal control over the models (structures) being viewed. A
change made by any participant is immediately propagated to all
other participants, so that a synchronized view of the data is
maintained throughout a session.
Because of the complex 3D nature of molecular models, inter-
active examination of models in a real-time collaborative environ-
ment is far more effective than traditional asynchronous forms of
communication, such as passing molecular data back and forth
through e-mail. A crucial element of real-time collaboration soft-
ware is the efficient transfer of information. Not only must the
software ensure that information is transmitted rapidly, it must also
consider the availability of network resources, such as bandwidth,
and transmit information in an efficient format so that the appli-
cation can run in parallel with other bandwidth-heavy applications
such as videoconferencing software. Given these considerations,
application-independent collaboration tools are not as responsive
for sharing molecular modeling sessions. Such desktop-sharing
applications (e.g., Microsoft NetMeeting and Virtual Network
Computing
40
) function by transmitting the contents of the screen
from the workstation running the application to all who are sharing
it. This can be bandwidth-intensive, especially for molecular
graphics, where most operations alter a large amount of screen
content. Instead, the Collaboratory works on a lower level, by
transmitting small messages describing just the data that has been
modified.
The Collaboratory utilizes a star architecture, with a central hub
connected to multiple nodes. Each node is a user running Chimera,
while the hub is a separately running application that acts as a
rendezvous point between the nodes. Participants’ instances of
Chimera are notified when a model has been opened, closed, or
modified. A data file need only be present on the system of the
participant who opens the file in Chimera. Parameters monitored
UCSF Chimera 1609