Q2. Why was the possibility to observe slow magnetic relaxation discarded?
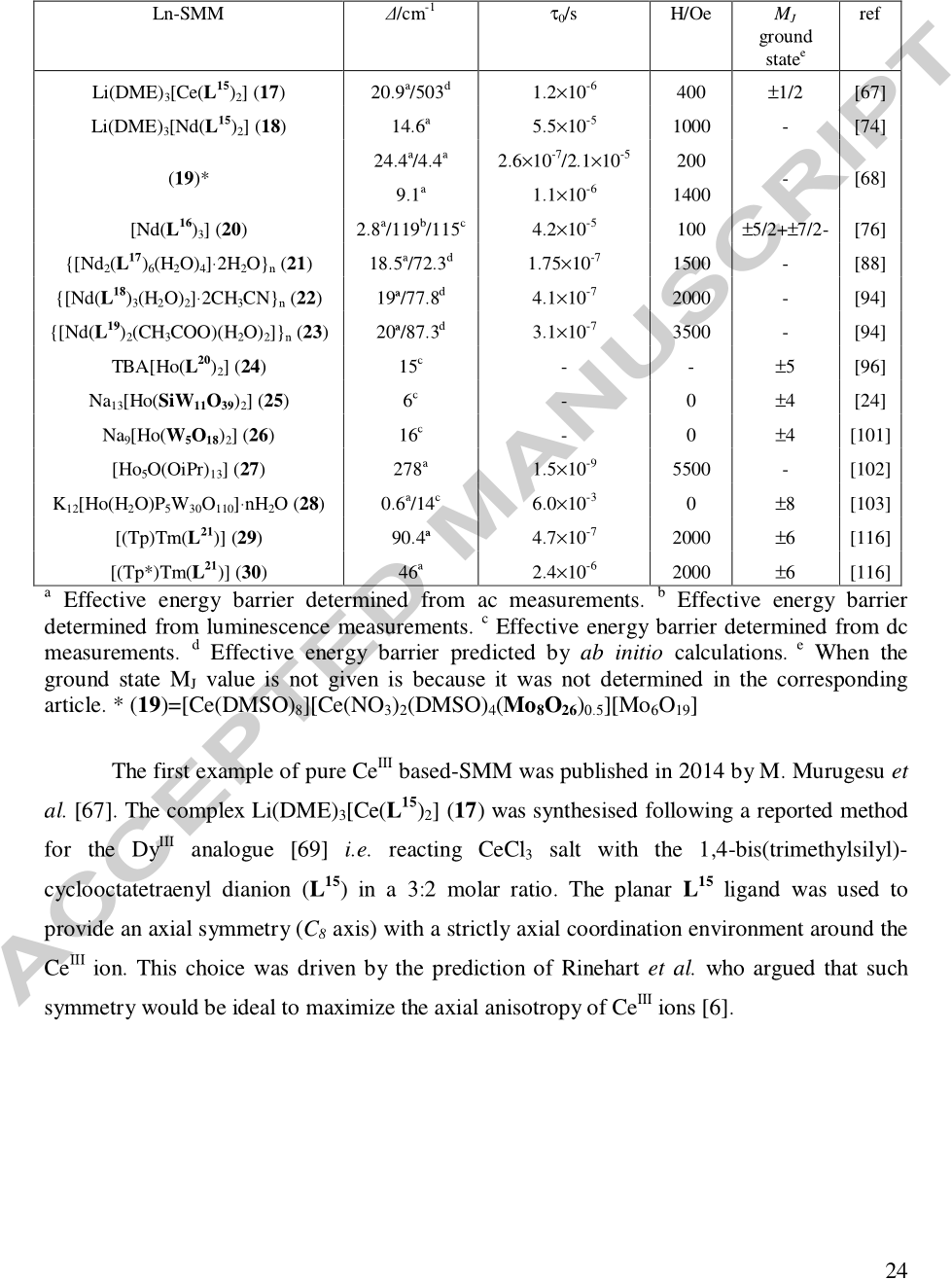
The possibility to observe slow magnetic relaxation for the Ce III ion in eight-coordination was discarded due to the low symmetry of the coordination sphere and the isotropic nature of the ligands composing the coordination sphere.
Q3. What is the reason for the rarity of Ho III -based SMMs?
One can take in his mind that the rarity of Ho III -based SMM can be due to the non-Kramers nature of such lanthanide, which places constraints on the symmetry of the coordination environment needed for SMM behaviour, and to the fact the 4f electron density is not very anisotropic.
Q4. What is the reason for the absence of SMM behaviour without external field?
The absence of SMM behaviour without external field wasattributed to the significant transversal g factors and the large tunnelling gap in the ground exchange doublet (0.01 cm -1 ) allowing a strong quantum tunnelling of the magnetization.
Q5. How long is the energy barrier for Yb-based SMM?
Above 3K, the extracted energy barrier of 19.5 K is one of the highest for Yb-based SMM while the relaxation time of 1.0×10-7 s is classical for such systems.
Q6. What is the molecule that stabilizes a S=10 spin ground-state?
In this molecule the antiferromagnetic interactions between spins of Mn III and Mn IV ions stabilize a S=10 spin ground-state which can be trapped in up (Ms = +S) or down (Ms = -S) orientations.
Q7. What was the method used to reproduce the static magnetic properties?
Attempts to reproduce the static magnetic properties were done using an extendedStevens operators technique taking into account both crystal field effects and possible ferromagnetic interactions (because of the increase of χMT vs. T curve below 9 K (Figure 9b)).
Q8. How many Oe field is required to determine the magnetic susceptibility?
As for almost all YbIII-based SMMs, no out-of-phase signal of the magneticsusceptibility is observed without applied magnetic field, whereas an optimal field of 1000
Q9. What is the first unusual lanthanide ion involved in a SMM?
The lanthanide mononuclear SMM started in 2003 with the association of a Dy III ionand double Decker ligands [95], while the first uncommon lanthanide ion involved in a SMM was HoIII in 2005 [96].
Q10. What is the equatorial ligand used for the lanthanide ?
Gao et al. associated an equatorial ligand such as COT ligand (L 21 , Scheme 3) in order to satisfy the Ising limit condition for a lanthanide ion with a prolate electron density.
Q11. What other strategies were already successful in obtaining SMM with lanthanide ions?
two other strategies to obtain SMM with this kind of lanthanide ions were already successful: i) the insertion of diamagnetic ions such as Zn II which add an electrostatic effect on the active centre and ii) the doping of the active SMM with a second 4f element.
Q12. How is the magnetic susceptibility of CeIII ion observed without a magnetic field?
As for 17, no out-of-phase signal of the magnetic susceptibility is observed without magnetic field while a weak field leads to its appearance (200 Oe).
Q13. What is the energy gap between the ground and first excited states?
The energy gap between the ground- and first excited-states was estimated to 130 cm-1 for 4 which is one order of magnitude higher than the energy gap found for the Er III and Dy III analogues.
Q14. What is the potential for the elaboration of SMM?
the NdIII ion which possesses a 4f3 electronic configuration could be also a potential candidate for the elaboration of SMM.
Q15. What is the nature of the first neighbouring atoms?
In other words, the nature of the first neighbouring atoms (oxygen vs. nitrogen) induces two different distortion of the D4d coordination polyhedron (compressed vs. elongated).
![Figure 3. (a) Molecular structure of [Yb(tta)3(L 2)] with experimental (orange) and theoretical (green) anisotropy axis. (b) The solid-state emission spectrum is given with an appropriate shift of energy scale. Correlation between the main contributions of the emission spectrum (black sticks) and the energy splitting of the 2 F7/2 multiplet ground-state obtained by MSCASPT2/RASSI-SO calculations (red sticks). Adapted from ref [46].](/figures/figure-3-a-molecular-structure-of-yb-tta-3-l-2-with-1d8azksp.png)

![Figure 10. Molecular structure of [Yb2(L 10)2(acac)2(H2O)]. Dichloromethane molecules of crystallization and hydrogen atoms are omitted for clarity. Adapted from ref [64].](/figures/figure-10-molecular-structure-of-yb2-l-10-2-acac-2-h2o-2f9ewbc7.png)
![Figure 25. (a) Molecular structures of ([Zn(L 24 )Yb(H2O)(CO3)]2)2 + and 35. Hydrogen atoms, anions and solvent molecules of crystallisation have been omitted for clarity. (b) Frequency dependences of the magnetic susceptibilities in the 2-5.5 K temperature range and Arrhenius plots (red line, blue line and black line are representative of QTM+Orbach, QTM+Raman and Orbach respectively). Adapted from ref [131].](/figures/figure-25-a-molecular-structures-of-zn-l-24-yb-h2o-co3-2-2-16tyt81i.png)
![Figure 19. (a) Molecular structure of [Ho(W5O18)2] 9- . Sodium cations and water molecule of crystallisation are omitted for clarity. (b) Temperature dependent high-frequency EPR spectrum for the Na9[Ho0.25Y0.75(W5O18)2] sample between 2.2 and 10 K. (c) From up to down: Experimental (blue line) and simulated (red line) X-band EPR spectra using the 9.64 GHz perpendicular excitation mode for Na9[Ho0.1Y0.9(W5O18)2], parallel excitation mode for Na9[Ho0.1Y0.9(W5O18)2], and perpendicular excitation mode for 26. Adapted from ref [101].](/figures/figure-19-a-molecular-structure-of-ho-w5o18-2-9-sodium-3sf0hif7.png)
![Figure 28. (a) Molecular structure of [Yb(L 27 )2] - . Hydrogen atoms and cation are omitted for clarity. (b) Arrhenius plots for complexes 40 and 42 under an applied field of 1800 Oe. Adapted from ref [135].](/figures/figure-28-a-molecular-structure-of-yb-l-27-2-hydrogen-atoms-ecfmrxh7.png)
![Figure 15. (a) Molecular structure of 20. Hydrogen atoms are omitted for clarity. (b) Arrhenius plots for different doping values of 20 in a diamagnetic matrix of the La III analogue. Dashed line is given for a pure Orbach process. Green lozenges, purple triangles, blue squares and red full circles are given for a doping of 3.8%, 15%, 59% and pure Neodymium compound. Adapted from ref [76].](/figures/figure-15-a-molecular-structure-of-20-hydrogen-atoms-are-30ao6hde.png)
![Figure 23. (a) Molecular structures of [YbZn4(L 22)4(py)4(DMF)4] 3+ (left) and [YbZn4(L 22 )4(iqn)4(DMF)4] 3+ (right). Hydrogen atoms, triflate anions and solvent molecules of crystallisation have been omitted for clarity. (b) Correlations of the different set of data for the energy levels of the 2 F7/2 ground state multiplet obtained from dc fit (i) ac fit (ii) and luminescence spectra (iii) for 31 (left and 32 (right). Adapted from ref [124].](/figures/figure-23-a-molecular-structures-of-ybzn4-l-22-4-py-4-dmf-4-2hv2dp2r.png)
![Figure 21. (a) Molecular structure of 28. Hydrogen atoms are omitted for clarity. (b) Temperature dependence of the out-of-phase component of the magnetic susceptibility in an applied field of 5.5 kOe. In inset, Temperature dependence of the in-phase component of the magnetic susceptibility in an applied field of 5.5 kOe. The applied frequencies ranged from 20 to 1200 Hz. Adapted from ref [103].](/figures/figure-21-a-molecular-structure-of-28-hydrogen-atoms-are-1x1ahlyp.png)
![Figure 18. (a) Molecular structure of [Ho(L 20 )2] - . Hydrogen and TBA + cation are omitted for clarity. (b) Field-dependence of the magnetization at different temperature and field scan rate for 2% of 24 in a diamagnetic matrix of TBA[Y(L 20 )2]. Adapted from ref [96].](/figures/figure-18-a-molecular-structure-of-ho-l-20-2-hydrogen-and-3lu4t9jy.png)
![Figure 4. Molecular structure of [Yb(L 3 )3] 3- . The tetraethylamonium cations and hydrogen atoms are omitted for clarity. Adapted from ref [50].](/figures/figure-4-molecular-structure-of-yb-l-3-3-3-the-3llpgq8w.png)
![Table 6. Heterobinuclear Lanthanide SMMs (Ln=DyIII and YbIII) DyYb-SMM ∆/cm-1 τ0/s H/Oe MJ ground statee ref [Yb2(L 26 )2Cl4(H2O)(MeCN)]⋅CH3CN (37) [Dy0.87Yb1.13(L 26 )2Cl4(H2O)(MeCN)]⋅CH3CN (39)](/figures/table-6-heterobinuclear-lanthanide-smms-ln-dyiii-and-ybiii-bdihp4dj.png)
![Figure 24. (a) Molecular structure of [Zn3Yb(L 23 )(NO3)3]. Hydrogen atoms and methanol molecules of crystallisation have been omitted for clarity. (b) Frequency dependence of the magnetic susceptibility between 1.8 and 5.5 K. Adapted from ref [129].](/figures/figure-24-a-molecular-structure-of-zn3yb-l-23-no3-3-hydrogen-3c6wdd01.png)
![Figure 17. Molecular structures of {[Nd(L 18 )3(H2O)2]}n (a) and 23 (b). Hydrogen atoms and solvent molecules of crystallisation have been omitted for clarity. Adapted from ref [94].](/figures/figure-17-molecular-structures-of-nd-l-18-3-h2o-2-n-a-and-23-3hu5p5i0.png)
