University of Groningen
Work function anisotropy and surface stability of half-metallic CrO(2)
Attema, J. J.; Uijttewaal, M. A.; de Wijs, G. A.; de Groot, R. A.
Published in:
Physical Review. B: Condensed Matter and Materials Physics
DOI:
10.1103/PhysRevB.77.165109
IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
it. Please check the document version below.
Document Version
Publisher's PDF, also known as Version of record
Publication date:
2008
Link to publication in University of Groningen/UMCG research database
Citation for published version (APA):
Attema, J. J., Uijttewaal, M. A., de Wijs, G. A., & de Groot, R. A. (2008). Work function anisotropy and
surface stability of half-metallic CrO(2).
Physical Review. B: Condensed Matter and Materials Physics
,
77
(16), [165109]. https://doi.org/10.1103/PhysRevB.77.165109
Copyright
Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the
author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons).
The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license.
More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne-
amendment.
Take-down policy
If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately
and investigate your claim.
Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the
number of authors shown on this cover page is limited to 10 maximum.
Download date: 10-08-2022

Work function anisotropy and surface stability of half-metallic CrO
2
J. J. Attema,
1
M. A. Uijttewaal,
1,
*
G. A. de Wijs,
1
and R. A. de Groot
1,2,†
1
ESM, IMM, Radboud University, Toernooiveld 1, 6525ED Nijmegen, The Netherlands
2
Zernike Institute for Advanced Materials, Nijenborgh 6, 9747AG Groningen, The Netherlands
共Received 8 October 2007; revised manuscript received 20 February 2008; published 4 April 2008
兲
Insight in the interplay between work function and stability is important for many areas of physics. In this
paper, we calculate the anisotropy in the work function and the surface stability of CrO
2
, a prototype half-
metal, and find an anisotropy of 3.8 eV. An earlier model for the relation between work function and surface
stability is generalized to include the transition-metal oxides. We find that the lowest work function is obtained
for surfaces with the most electropositive element, whereas the stable surfaces are those containing the element
with the lowest valency. Most CrO
2
surfaces considered remain half-metallic, thus the anisotropy in the work
function can be used to realize low resistance, half-metallic interfaces.
DOI: 10.1103/PhysRevB.77.165109 PACS number共s兲: 73.30.⫹y, 75.30.Gw, 72.25.Mk, 73.20.At
I. INTRODUCTION
Electron-emitting materials are applied in many estab-
lished areas of technology, for example, vacuum electronic
devices such as cathode-ray tubes, microwave devices, and
free electron lasers. They are also of interest in emerging
technologies such as organic light emitting diodes and spin-
tronics, which can benefit from an understanding of the work
function.
An important aspect of the electron-emitting properties of
the cathode material is the work function. The lifetime of the
device is related to the surface stability and the applied volt-
age. This often implies that cathodes need to have both a low
work function and a high surface stability. At first, these
requirements appear to be incompatible: A low work func-
tion means loosely bound electrons, implying a less stable
surface. This reasoning holds for the elements. For instance,
cesium has a low work function 共2.14 eV兲 but it is highly
reactive, whereas gold is stable but has a high work function
共5.1 eV兲.
1
Experimental results for alloys suggest the alloy
effect: The work function and surface stability interpolate
between those of the constituting elements.
2
However, recent
theoretical work has shown a different picture for intermetal-
lic compounds. If a compound allows the formation of a
surface of nonstoichiometric composition and charge transfer
occurs, surfaces with a resulting surface dipole are possible.
This surface dipole, depending on its orientation, raises or
lowers the work function. The work function may be lowered
to even below the work functions of the constituting ele-
ments. This was first demonstrated in a computational study
for BaAl
4
.
3
The barium terminated 共001兲 surface has a work
function of 1.95 eV, which is lower than that of elemental
barium 共2.32 eV兲. It is even lower than that of any element,
which is clearly in contradiction with the alloy effect. It is
important to notice that the work function for polar com-
pounds, i.e., compounds containing atoms with different
electronegativities, is expected to show a large anisotropy, as
the surface dipole depends on surface orientation. For BaAl
4
and similar compounds, the surface with the lowest work
function was calculated to be the most stable as well. This
was explained by the lower electronegativity of barium.
4,5
The following model was formulated: For an intermetallic
compound with polar surfaces, the difference in electronega-
tivity determines the work function, and the most stable sur-
face has the lowest work function.
Electron injection is also important for spin injection, i.e.,
spintronics. Spintronics aims to integrate the control of spin
degrees of freedom with the conventional charge based elec-
tronics. For spin injection, a source of spin polarized elec-
trons is needed. Materials considered for spin injection are
half-metals, as they intrinsically have 100% spin polariza-
tion. Work on spin injection further focuses on obtaining a
spin polarization as high as possible at surfaces and
interfaces.
6,7
Recently, the importance of electrical band en-
gineering for spin injection has become apparent.
8,9
Ideally,
the states carrying the current on either side of the interface
are aligned. However, in practice, there is a difference in
chemical potential 共see Fig. 1兲. This difference in potential
causes a barrier at the interface and reduces the electrical
efficiency of the spin injection. Although an interface is more
complex than two surfaces, some properties of the two indi-
vidual surfaces carry over to the interface. In a first approxi-
mation, the height of the interface barrier is related to the
work function of the two separate surfaces.
10
For a given
half-metal/semiconductor interface, the anisotropy in work
FIG. 1. A schematic drawing of the energy levels of an electron
injector/semiconductor interface. Filled and empty states are shaded
dark and light gray, respectively. The work function of the injector
共⌽兲 is the difference between the chemical potential in the bulk and
the vacuum potential. A mismatch in the chemical potential of the
injector and conduction band of the semiconductor results in a po-
tential barrier at the interface 共⌬V兲.
PHYSICAL REVIEW B 77, 165109 共2008兲
1098-0121/2008/77共16兲/165109共9兲 ©2008 The American Physical Society165109-1

function can be used to minimize the potential barrier.
We will extend the applicability of the model and include
materials that are of interest for spintronic applications:
transition-metal oxides. In this paper, we investigate the an-
isotropy in the work function and the surface stability of
ferromagnetic CrO
2
. CrO
2
is widely studied; it is a half-
metal in calculations and it has experimentally shown a very
high spin polarization.
11
The main difference between inter-
metallics and transition-metal oxides is in the combination of
electronegativity and valency. For intermetallic compounds,
the most electropositive atom also has the lowest valency,
resulting in stable, low work function surfaces. For
transition-metal oxides, the situation is reversed: The lowest
valency occurs almost always for the most electronegative
atom, in this case oxygen. Another difference between
transition-metal oxides and the previously studied com-
pounds is the occurrence of magnetism. They will provide a
challenging test for the model.
This paper is organized as follows. First, we describe the
computational method. Then results on bulk CrO
2
are briefly
discussed. Results on the structural relaxation are presented,
followed by the work functions and surface stabilities, and
an outlook.
II. COMPUTATIONAL METHOD
The calculations were carried out using density functional
theory with the PW91 generalized gradient approximation
functional.
12,13
We employed projector augmented plane
waves
14,15
as implemented in the Vienna ab initio simulation
package 共VASP兲.
16–18
The kinetic energy cutoff was set to
400 eV. The Brillouin zone was sampled with a Monkhorst–
Pack mesh with a 6⫻6⫻8 grid for bulk CrO
2
,1⫻6⫻8 for
the 共100兲 surfaces, 1⫻4⫻8 for the 共110兲 surfaces, and 7
⫻7⫻1 for the 共001兲 and 共011兲 surfaces. The work functions
and surface stabilities were calculated using a supercell ap-
proach. The supercell contained slabs with thicknesses of six
bulk unit cells for 共001兲, 共100兲, and 共011兲, and eight bulk unit
cells for 共110兲, and at least 10 Å of vacuum. We used a
minimal unit cell in the directions parallel to the surface.
Surface reconstructions involving more than one unit cell or
the formation of a Cr
2
O
3
surface was not considered. The
surfaces at both sides of the slab were taken identical; there-
fore, some slabs are nonstoichiometric. During relaxation,
the central region of the slab was held fixed to obtain faster
convergence.
III. BULK CrO
2
Experimentally, CrO
2
is a ferromagnet with a Curie tem-
perature of 386 K.
19
The half-metallic character of CrO
2
and
several CrO
2
surfaces 共100 and 110兲 has been shown using
spin-resolved photoemission,
20,21
x-ray absorption,
22,23
opti-
cal spectroscopy,
24
and point contact Andreev reflection.
25
Earlier photoemission measurements found a small intensity
near E
F
only, but this was probably due to surface disorder or
the formation of Cr
2
O
3
at the surface.
20
Basically, CrO
2
is an ionic compound containing Cr
4+
and
O
2−
. It has a magnetic moment of 2
B
/ f.u., located almost
entirely on the chromium atoms. The half-metallic property
of CrO
2
is mainly caused by its chemical composition, i.e.,
the chromium valency, rather than the crystal structure. CrO
2
is a strong magnet, the chromium magnetic moment does not
depend on the size of the exchange splitting, as can be seen
from the density of states in Fig. 3.
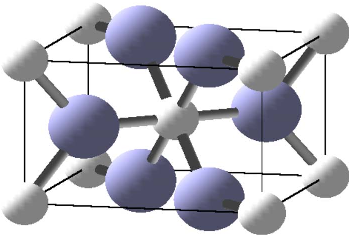
The crystal structure of CrO
2
is depicted in Fig. 2.It
crystallizes in the rutile structure, space group P4
2
/ mnm
共No. 136兲, with experimental lattice parameters a
=4.4218 Å and c =2.9182 Å. The chromium is at position
2a, oxygen is at position 4f with parameter x =0.301.
26
The
chromium atoms are almost perfectly octahedrally sur-
rounded by oxygen atoms, with Cr-O distances of 1.90 and
1.89 Å; each oxygen atom has three chromium neighbors.
The calculated electronic structure of bulk CrO
2
has been
extensively studied before.
27,28
Special attention has been
given to the importance of correlation effects.
29,30
Because
we are interested in structural optimizations and work func-
tions, i.e., electrostatics, local density approximation 共LDA兲
is adequate. In view of the comparison between LDA and
LDA+U and the experiment made in Ref. 29,wedonot
expect that the latter performs better for our purposes. After
relaxation of the lattice parameters and the positional param-
eter of the oxygen atoms, we found a =4.405 Å, c
=2.905 Å with the oxygen at position 4f, and x =0.303. The
calculated parameters agree with the experimental values
FIG. 2. 共Color online兲 A CrO
2
unit cell. Oxygen atoms are large
共blue兲, while chromium atoms are small 共white兲.
-4
-2
0
2
4
-6 -4 -2 0 2
states / unit cell eV
E-E
F
(eV)
FIG. 3. Calculated density of states for CrO
2
.
ATTEMA et al. PHYSICAL REVIEW B 77, 165109 共2008兲
165109-2

共within 0.5%兲 and they will be used in this paper. For con-
venience, we show the calculated density of states in Fig. 3.
It shows the crystal field splitting of the chromium 4d band.
As the chromium atoms have an octahedral coordination, its
d band splits into a threefold degenerate t
2g
band and a dou-
bly degenerate e
g
band. The t
2g
band shows additional struc-
ture due to the deviation from perfect octahedral symmetry.
In the minority spin direction, the exchange interaction shifts
the chromium 4d band completely above the Fermi level and
opens a band gap.
IV. SURFACES OF CrO
2
Although bulk CrO
2
is a half-metal, it is not a priori clear
that surfaces of CrO
2
should be half-metallic. For NiMnSb,
the first discovered and, consequently, the most extensively
studied half-metal, surfaces and interfaces are generally not
half-metallic.
31
The half-metallic character of NiMnSb is a
consequence of the specific symmetry in the bulk. This sym-
metry is destroyed at the interface and, therefore, the half-
metallic character is lost; only with careful engineering can
half-metallic interfaces be constructed.
7
However, for CrO
2
,
surfaces will be half-metallic as long as the chromium va-
lency is conserved. Indeed, earlier calculations for the 共001兲
surface showed that the half-metallic character was
maintained.
32,33
In this section, we will first describe in detail the calcu-
lated surfaces, both before and after structural relaxation, and
we will compare with the literature where available. At the
end of the section, general conclusions will be presented.
A. (100) surfaces
Three different 共100兲 surfaces can be constructed: One
surface containing a chromium atom 共100 Cr兲, one surface
terminating with a single oxygen layer 共100 O兲, and one
surface terminating with two oxygen layers 共100 OO兲共see
Fig. 4兲.
For the 共100 Cr兲 surface, the chromium in the first layer
shifts 0.11 Å inward. It has only three oxygen neighbors and,
after relaxation, the nearest neighbor distance is 1.80 Å on
average. The oxygen atoms move −0.28 and 0.15 Å along
关010兴, and 0.61 and 0.24 Å outward for the second and fifth
layers. The third layer moves 0.13 Å outward. The relaxed
structure agrees with the calculations reported by Hong and
Che
33
Upon relaxation of the 共100 O兲 surface, chromium atoms
in the second layer shift −0.10 Å along 关010兴. The second
and fifth layers also shift 0.10 Å outward. The oxygen atoms
shift 0.24 and 0.16 Å along 关010兴, and 0.20 and 0.28 Å out-
ward for the first and third layers, respectively. Compared to
that of Hong and Che, the relaxation parallel to the surface is
similar, but our shift perpendicular to the surface is larger.
For the 共100 OO兲 surface, the first oxygen moves 0.18 Å
outward and the oxygens in the fourth layer move 0.14 Å
outward. The chromium atoms in the third layer move
0.41 Å outward and 0.15 Å along 关010兴, while the chromium
atoms in the sixth layer move 0.14 Å outward. The oxygen
atom in the top layer has only one chromium neighbor and,
as a result, the Cr-O distance after relaxation is reduced to
1.59 Å.
B. (001) surface
In the 关001兴 direction, only one termination is possible
共see Fig. 5兲. The surface is stoichiometric, containing one Cr
and two O atoms. The oxygen atoms in the top layer have
(100 Cr) (100 O) (100 OO)
FIG. 4. 共Color online兲 A view along 关001兴 of the relaxed 共100兲 surfaces. The top of the figure is the surface facing the vacuum, while the
bottom is toward the bulk. Oxygen atoms are large 共blue兲, while chromium atoms are small 共white兲.
(001)
FIG. 5. 共Color online兲 A view along 关100兴 of the relaxed 共001兲
surface. The top of the figure is the surface facing the vacuum,
while the bottom is toward the bulk. Oxygen atoms are large 共blue兲,
while chromium atoms are small 共white兲.
WORK FUNCTION ANISOTROPY AND SURFACE… PHYSICAL REVIEW B 77, 165109 共2008兲
165109-3

lost one chromium neighbor, while the chromium has four
oxygen neighbors. After relaxation, the chromium atoms
move 0.15 Å inward and 0.23 Å outward for the first and
second layers, respectively. The oxygen atoms in the first
layer move 0.31 Å outward and 0.23 Å along 关110兴 toward
the nearest chromium atom. The Cr-O distance for the sur-
face oxygens is 1.72 Å.
C. (110) surfaces
In the 共110兲 direction, there are again three different ter-
minations. One containing two oxygen and two chromium
atoms 共110 CrO兲, and two surfaces containing one oxygen
关the 共110 O兲 and 共110 OO兲 surfaces兴共see Fig. 6兲.
After relaxation of the 共110 CrO兲 surface, the fivefold
surrounded chromium atom in the top layer moves 0.16 Å
outward, while the fourfold surrounded chromium atom
moves 0.05 Å inward. The oxygen atoms in the first layer
move 0.51 Å outward. The second and third oxygen layers
move 0.10 Å and 0.21 Å outward.
Adding another oxygen layer gives the 共110 O兲 surface.
Upon relaxation, the oxygen in the first layer moves 0.10 Å
outward. The oxygens in the second layer move 0.24 Å out-
ward. The second layer also contains two chromium atoms,
one with five oxygen neighbors and one with six neighbors.
The sixfold surrounded chromium moves 0.27 Å outward,
while the fivefold surrounded chromium moves slightly in-
ward. The third layer oxygen moves 0.13 Å outward.
Finally, the 共110 OO兲 surface is obtained by adding an-
other oxygen layer. All chromium atoms have a bulklike six-
fold coordination, but the first two oxygen layers have miss-
ing neighbors. The first layer oxygen atom has only one
neighboring chromium, while the second layer oxygen atoms
has two. The oxygens in the first layer relax 0.11 Å outward.
In the third layer, one chromium moves 0.41 Å outward,
reducing the distance with the first layer oxygen to 1.59 Å;
the other chromium moves 0.15 Å outward.
D. (011) surfaces
In the 共011兲 direction 共see Fig. 7兲, CrO
2
consists of planes
containing either two oxygen or two chromium atoms. There
are three possible terminations: a chromium terminated sur-
face 共011 Cr兲, one with a single oxygen layer 共011 O兲, and
one with a double oxygen layer 共011 OO兲.
For the 共011 O兲, the relaxation has only a small effect.
The chromium atoms in the second layer only have five near-
est oxygen atoms; they relax slightly outward and move
0.16 Å along 关100兴. The oxygens in the first layer are also
missing a neighbor; they move a little inward and −0.08 Å
along 关100兴. The final Cr-O distance at the surface is 1.81 Å.
In the 共011 OO兲 surface, the first layer oxygens have only
one chromium neighbor. They move 0.23 Å along 关011兴 and
0.07 Å inward, reducing the Cr-O distance to 1.59 Å. The
(110 CrO) (110 O) (110 OO)
FIG. 6. 共Color online兲 A view along 关001兴 of the relaxed 共110兲 surfaces. The top of the figure is the surface facing the vacuum, while the
bottom is toward the bulk. Oxygen atoms are large 共blue兲, while chromium atoms are small 共white兲.
(011 Cr) (011 O) (011 OO)
FIG. 7. 共Color online兲 A view along 关100兴 of the relaxed 共011兲 surfaces. The top of the figure is the surface facing the vacuum, while the
bottom is toward the bulk. Oxygen atoms are large 共blue兲, while chromium atoms are small 共white兲.
ATTEMA et al. PHYSICAL REVIEW B 77, 165109 共2008兲
165109-4