')9015/:65&51<-891:>$+06636.-,1+15-')9015/:65&51<-891:>$+06636.-,1+15-
1/1:)3644659-+2-81/1:)3644659-+2-8
!7-5++-99";*31+):1659
+69:*-5-@:)5)3>9196./6=5;9-15+65:863315/<)5+64>+15+69:*-5-@:)5)3>9196./6=5;9-15+65:863315/<)5+64>+15
8-919:)5:5:-86+6++;9:8)59419916591:=68:0:0-781+-8-919:)5:5:-86+6++;9:8)59419916591:=68:0:0-781+-
);8)";?51)2
$)15:6;19&51<-891:>
):03--5 133-971-
$)15:6;19&51<-891:>
%-88>--:
$)15:6;19&51<-891:>
)815633-.
')9015/:65&51<-891:>$+06636.-,1+15-15$:6;19
15,);5,>
')9015/:65&51<-891:>$+06636.-,1+15-15$:6;19
6336=:019)5,),,1:165)3=6829):0::79,1/1:)3+644659=;9:3-,;67-5()++-99(7;*9
")8:6.:0--,1+15-)5,-)3:0$+1-5+-9644659
#-+644-5,-,1:):165#-+644-5,-,1:):165
";?51)2);8)133-971-):03--5 --:%-88>633-.)815)5,;5,>15,)+69:*-5-@:
)5)3>9196./6=5;9-15+65:863315/<)5+64>+158-919:)5:5:-86+6++;9:8)59419916591:=68:0:0-
781+-5.-+:16565:863)5,6971:)371,-41636/>
0::79,1/1:)3+644659=;9:3-,;67-5()++-99(7;*9
%019!7-5++-99";*31+):16519*86;/0::6>6;.68.8--)5,67-5)++-99*>1/1:)3644659-+2-8:0)9*--5
)++-7:-,.6815+3;916515!7-5++-99";*31+):1659*>)5);:0681?-,),41519:8):686.1/1:)3644659-+2-8
68468-15.684):16573-)9-+65:)+:<)5)4=;9:3-,;

A Cost–Benefit Analysis of Gown Use in Controlling Vancomycin‐Resistant Enterococcus
Transmission: Is It Worth the Price?•
Author(s): Laura A. Puzniak , PhD, Kathleen N. Gillespie , PhD, Terry Leet , PhD, Marin Kollef
, MD, Linda M. Mundy , MD
Reviewed work(s):
Source:
Infection Control and Hospital Epidemiology,
Vol. 25, No. 5 (May 2004), pp. 418-424
Published by: The University of Chicago Press on behalf of The Society for Healthcare Epidemiology of
America
Stable URL: http://www.jstor.org/stable/10.1086/502416 .
Accessed: 28/03/2012 16:37
Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at .
http://www.jstor.org/page/info/about/policies/terms.jsp
JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of
content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms
of scholarship. For more information about JSTOR, please contact support@jstor.org.
The University of Chicago Press and The Society for Healthcare Epidemiology of America are collaborating
with JSTOR to digitize, preserve and extend access to Infection Control and Hospital Epidemiology.
http://www.jstor.org

418 INFECTION CONTROL AND HOSPITAL EPIDEMIOLOGY May 2004
A C
OST–BENEFIT ANALYSIS OF GOWN USE IN
C
ONTROLLING VANCOMYCIN-RESISTANT
E
NTEROCOCCUS
TRANSMISSION: IS IT WORTH THE PRICE?
Laura A. Puzniak, PhD; Kathleen N. Gillespie, PhD; Terry Leet, PhD; Marin Kollef, MD; Linda M. Mundy, MD
Enterococci are the third most common pathogen
associated with nosocomial infections, accounting for 12%
of intensive care unit infections.
1
The increasing preva-
lence of enterococcal infections is problematic due to lim-
ited treatment and eradication strategies. Furthermore,
the public health threat from vancomycin-resistant ente-
rococci (VRE) is more imminent given the recent detec-
tion of vancomycin-resistant Staphylococcus aureus
(VRSA).
2-4
The presence of vanA in a clinical isolate of
VRSA from a host colonized with VRE suggests exchange
of genetic material between these gram-positive
pathogens.
2
Hospital Infection Control Practices Advisory
Committee guidelines for controlling VRE include screen-
ing high-risk populations, using vancomycin appropriately,
educating medical staff, and implementing infection con-
trol procedures.
5
Recommended infection control prac-
tices include the use of gloves and gowns with patients col-
onized or infected with drug-resistant pathogens.
5
Despite
encouraging results for the efficacy of gown use, there is
ongoing debate over the cost versus benefit of requiring
gown use to prevent VRE transmission.
6-15
Few studies have assessed the costs and benefits
associated with gown use.
11-13
One study reported an
annual cost increase of $11,303 for gowns and gloves after
a VRE epidemic began.
12
The authors concluded that pre-
venting a case of VRE bacteremia was worth the addition-
al cost for implementing isolation precautions. In contrast,
a study in a bone marrow transplant unit reported that dis-
continuing the use of gowns and shoe covers created a
$70,000 savings for the unit with no increase in infection
rates.
11
Our prior work showed that requiring healthcare
workers and visitors to wear gowns when entering the
rooms of patients in a medical intensive care unit (MICU)
reduced the patients’ risk of VRE acquisition during peri-
ods of high VRE colonization pressure.
13
The purpose of
this study was to determine the costs and benefits of this
enhanced infection control program aimed at reducing
VRE transmission.
Drs. Puzniak, Leet, and Mundy are from the Department of Community Health and Dr. Gillespie is from the Department of Health
Management and Policy, Saint Louis University School of Public Health, St. Louis, Missouri. Drs. Kollef and Mundy are from the Department of
Medicine, Washington University School of Medicine, St. Louis, Missouri.
Address reprint requests to Linda M. Mundy, MD, Washington University School of Medicine, 660 S. Euclid Avenue, Campus Box 8051, St.
Louis, MO 63110.
The authors thank the 65 members of the medical intensive care unit, the Barnes–Jewish Hospital medicine residents, and the Washington
University faculty who participated in the care of these patients; Donna Prentice and Jennie Mayfield for managing the VRE surveillance program;
Joan Hoppe-Bauer for providing microbiology data; and Dr. Brooke Shadel for her insightful comments during manuscript review.
OBJECTIVE: To determine the net benefit and costs
associated with gown use in preventing transmission of van-
comycin-resistant Enterococcus (VRE).
DESIGN: A cost–benefit analysis measuring the net ben-
efit of gowns was performed. Benefits, defined as averted costs
from reduced VRE colonization and infection, were estimated
using a matched cohort study. Data sources included a step-down
cost allocation system, hospital informatics, and microbiology
databases.
SETTING: The medical intensive care unit (MICU) at
Barnes–Jewish Hospital, St. Louis, Missouri.
PATIENTS: Patients admitted to the MICU for more than
24 hours from July 1, 1997, to December 31, 1999.
INTERVENTIONS: Alternating periods when all health-
care workers and visitors were required to wear gowns and
gloves versus gloves alone on entry to the rooms of patients col-
onized or infected with VRE.
RESULTS: On base-case analysis, 58 VRE cases were
averted with gown use during 18 months. The annual net benefit
of the gown policy was $419,346 and the cost per case averted of
VRE was $1,897. The analysis was most sensitive to the level of
VRE transmission.
CONCLUSIONS: Infection control policies (eg, gown
use) initially increase the cost of health services delivery.
However, such policies can be cost saving by averting nosocomi-
al infections and the associated costs of treatment. The cost sav-
ings to the hospital plus the benefits to patients and their families
of avoiding nosocomial infections make effective infection con-
trol policies a good investment (Infect Control Hosp Epidemiol
2004;25:418-424).
ABSTRACT

Vol. 25 No. 5 COST–BENEFIT ANALYSIS OF GOWNS 419
METHODS
Study Population
All patients staying more than 24 hours in a 19-bed
MICU at Barnes–Jewish Hospital from July 1, 1997, to
December 31, 1999, were eligible. All healthcare workers
and visitors were required to wear gowns and gloves on
entry into the rooms of patients colonized or infected with
VRE from July, 1, 1997, to June 30, 1998, and from July 1,
1999, to December 31, 1999. During the 12 months
between these two periods, gowns were not required. The
institutional review board committees of Saint Louis
University and Washington University approved this
study.
During the entire study period, all patients were
actively screened for VRE by collection of stool for cul-
tures or rectal swabs on admission, every 7 days, and at
discharge from the MICU. Per hospital protocol, stool
specimens sent for the detection of Clostridium difficile
toxin were also screened for VRE. For each patient with
VRE, a sign requiring contact precautions and an isolation
cart containing a dedicated stethoscope, a glass ther-
mometer, and gloves were placed at the entrance to the
patient’s room. Contact precautions were continued
unless a patient had two subsequent consecutive stool
surveillance specimens that tested negative. Gowns that
were fluid resistant and laundered after each use were
added to the isolation cart during the designated gown
periods.
A matched cohort study design was used to deter-
mine the attributable cost of VRE. Patients without VRE
from the same MICU population were matched to patients
with VRE by diagnosis-related group (DRG) code, Acute
Physiology and Chronic Health Evaluation (APACHE) II
16
severity of illness score (± 2 points), and age (± 5 years).
17
One patient without VRE was randomly selected for each
patient colonized with VRE when there were multiple
patients without VRE with the same matching criteria.
Two patients without VRE were randomly selected, using
the same matching criteria, for each patient with VRE bac-
teremia. Two matched controls were used to increase sta-
tistical power due to the small number of patients with
VRE bacteremia. Four patients colonized with VRE and
two patients with VRE bacteremia were excluded from the
study population because there was not a match of a
patient without VRE.
Clinical endpoints were obtained from the hospital’s
informatics system. These included MICU and hospital
lengths of stay, presence of nosocomial bacteremia due to
oxacillin-resistant S. aureus (ORSA) or Pseudomonas
aeruginosa, and presence of colitis or diarrhea associated
with C. difficile toxin. The three nosocomial pathogens
were used to determine whether the frequency of co-
infections was similar between patients with and patients
without VRE.
Costs
Overall costs for the VRE surveillance and infection
control program were estimated using the hospital’s step-
down cost allocation system, which recorded line-item
cost data per resource consumed and total cost per hospi-
tal admission. MICU costs were estimated from these
data by dividing the patient’s total hospitalization cost by
total days of hospitalization and then multiplying the quo-
tient by the patient’s total MICU-days. This data system
also provided hospital reimbursement data, type of insur-
ance, case mix index, and DRG. Medicare patients from
the study population were used to determine the average
non-reimbursed hospitalization cost by VRE status.
The cost for each isolation cart included all initial
supplies. In addition to the costs for gowns, the costs
resulting from staff time to comply with gown use were
estimated. Observational time trials were used to estimate
the time required for healthcare workers to retrieve, don,
doff, and properly dispose of gowns. On three separate
occasions, two unobtrusive observers measured the
amount of time required by 128 healthcare workers to
comply with the gown policy. Our observations showed
that the average worker needed 60 seconds (range, 35 to
95 seconds) to don and doff gowns, which was similar to
the amount of time needed for the same activities in anoth-
er study.
18
To estimate the cost associated with excess
workload per VRE patient contact, the average time was
multiplied by the average registered nurse salary (exclud-
ing fringe benefits). Because a range of healthcare work-
ers entered a patient’s room, the average registered
nurse’s salary was used to approximate this cost.
Microbiology costs for each patient were obtained
from line-item reports from the hospital’s microbiology
database. Microbiology costs were inclusive of all related
testing costs (ie, materials, technician time, nursing time
for culture procurement, and overhead). Individualized
costs associated with contact precautions and surveil-
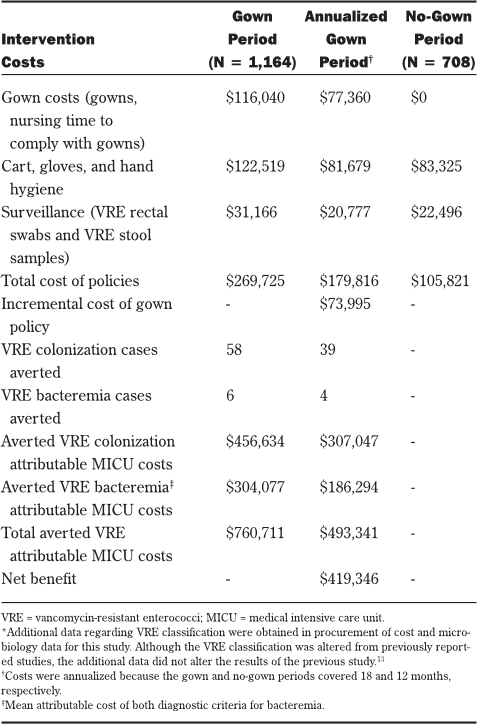
lance are listed in Table 1. All costs were reported in U.S.
dollars.
Decision Analysis
An event pathway of the study was constructed
showing VRE colonization and infection rates during this
30-month study period (Figure).
13
Costs were allocated to
each arm based on actual resources consumed per
patient. Each patient with VRE, regardless of study peri-
od, was charged the costs for a cart, gloves, and hand
hygiene. During the gown period, patients with VRE were
charged additional costs for gowns and nursing time to
comply with the gown policy.
Benefits were measured as the number of VRE
cases and the MICU costs averted. The number of VRE
cases averted was estimated by multiplying the difference
in the VRE rates between the study periods by the num-
ber of patients in the gown period. The number of VRE
cases averted per 1,000 MICU-days was calculated by tak-
ing the number of cases averted and dividing it by the
total number of MICU patient-days in the gown period and
multiplying by 1,000. Averted attributable cost for the
gown period and net benefit of the gown policy
19
were
computed as shown in equations 1 and 2, respectively.

420 INFECTION
CONTROL AND H
OSPITAL
EPIDEMIOLOGY
May 2004
(1) averted attributable cost
gown
= (attributable cost
of VRE colonization ⫻ annualized number of VRE colo-
nized cases averted) + (weighted mean attributable cost
of both diagnostic criteria of VRE bacteremia ⫻ annual-
ized number of VRE bacteremic cases averted)
(2) net benefit = averted attributable cost
gown
-
(annualized isolation/surveillance cost
gown
- isolation/sur-
veillance cost
no-gown
)
The latter term in equation 2 measured the incre-
mental costs of the gown policy. Costs and benefits in the
gown period were annualized because the gown period
was 6 months longer than the no-gown period.
Sensitivity Analysis
A sensitivity analysis was performed varying sever-
al parameters related to the assumptions regarding the
number of gowns used, time required to don and doff
gowns, VRE transmission rates for this analysis, and cost
of materials. Our baseline estimate for the number of
gowns used was 100 gowns per patient per day. We used
the previously reported value of 60 contacts per day
18
for
the lower limit and an equivalent difference, 140 contacts
per day, for the upper limit. The average number of VRE
cultures performed during the study was 2 per patient per
MICU stay. To adjust for variation in surveillance mecha-
nisms,
20-23
we used 2 cultures as the baseline measure and
varied this measure between 1 and 4 cultures per MICU
stay. Because there were differences in cost between pos-
itive and negative cultures, we used the same proportion
of positive cultures as the original analyses when the para-
meter was changed to 4 cultures per patient. Because the
costs of isolation materials and laboratory testing can dif-
fer between hospitals and can increase due to inflation, we
altered the costs for these items by 20% in both directions.
As shown in the figure, the risks of both acquiring VRE
and developing bacteremia were lower in the gown peri-
od; therefore, we altered the probability of acquiring VRE
in the gown period from 40% to 100% of the probability of
acquiring VRE in the no-gown period to determine the net
benefit at varying levels of VRE transmission.
Statistical Analysis
Univariate statistics were obtained using SPSS soft-
ware (version 10.0; SPSS, Inc., Chicago, IL). Differences
in characteristics between patients with and patients with-
out VRE were identified using t tests for continuous
covariates and chi-square tests for categorical covariates.
A Decision Tree Add-In for Microsoft Excel was used for
the decision analysis (TreePlan, version 1.62; Microsoft
Corp., Redmond, WA).
RESULTS
Matched Cohort
Based on the matching criteria, patients with and
patients without VRE were closely matched. The mean
APACHE II scores were similar between patients colo-
nized with VRE and their matched controls and between
patients with VRE bacteremia and their matched controls
(22.0 vs 21.8 and 26.5 vs 26.3, respectively), as were the
mean ages (62.3 vs 62.2 years and 65.4 vs 64.0 years,
respectively). In addition, there were no significant differ-
ences in the frequency of co-infections between patients
TABLE 1
INDIVIDUALIZED
COSTS ASSOCIATED WITH CONTACT PRECAUTIONS
AND VANCOMYCIN-RESISTANT ENTEROCOCCI SURVEILLANCE IN THE
M
EDICAL INTENSIVE CARE UNIT
Cost
Variable Cost per Day
Gown $0.75 each $75.00
Gloves $0.07/pair $7.00
Hand hygiene $0.10/use $10.00
Nursing time to don and doff gowns $27.00/hour $45.00
Isolation cart set up (cost of initial $18.00 One-time
cart set up—bag of gowns, cost
stethoscope, thermometer, and
box of gloves)
VRE-negative test $12.13 Varies
VRE-positive test $24.29 Varies
VRE = vancomycin-resistant enterococci.
89
VRE colonization from admission culture
94 95%
VRE + on admission
8%
5
VRE infection from admission culture
5%
1164
Admission gown period
62%
1011
No acquisition
94%
1070
VRE - admission 55
VRE acquired colonization
92%
59 93%
VRE acquisition
6%
4
1872 VRE acquired infection
Enter
1 7%
79
VRE colonization from admission culture
88 90%
VRE + on admission
12%
9
VRE infection from admission culture
10%
708
Admission no gown period
38%
552
No acquisition
89%
620
VRE - admission 62
VRE acquired colonization
88%
68 91%
VRE acquisition
11%
6
VRE acquired infection
9%
FIGURE. An event pathway showing vancomycin-resistant enterococci
(VRE) colonization and infection rates from July 1, 1997, to December 31,
1999, for patients in the medical intensive care unit.