




read more










In this work, the authors use slices that are 0.25 nm thick, much thinner than slices used in previous studies6,17,22-24,33, but the authors are able to obtain good statistics due to the long simulation times employed.
The first conclusion to draw is that the intrinsic profiles are independent of system size, which means that their method of calculation gives reproducible results.
In other words, the presence of the interface induces closer packing and antiparallel alignment of the nitrobenzene molecules, and this effect extends significantly towards the bulk organic region.
In their study of the water/CO2 interface, da Rocha et al. 25 used the mean square deviation of the interface location calculated at L/N = 0.7 nm to estimate the capillary wave width and, assuming ξ=0.9 nm, obtained interfacial tensions in good agreementwith γV.
For each configuration in the sampling stage, the authors rescaled the z coordinates of each atom by a fixed amount so that the center of mass of the organic phase is located at the origin.
The presence of an interface strongly affects the molecular organization of both phases,which becomes evident when one computes different properties as a function of the distance to the interface.
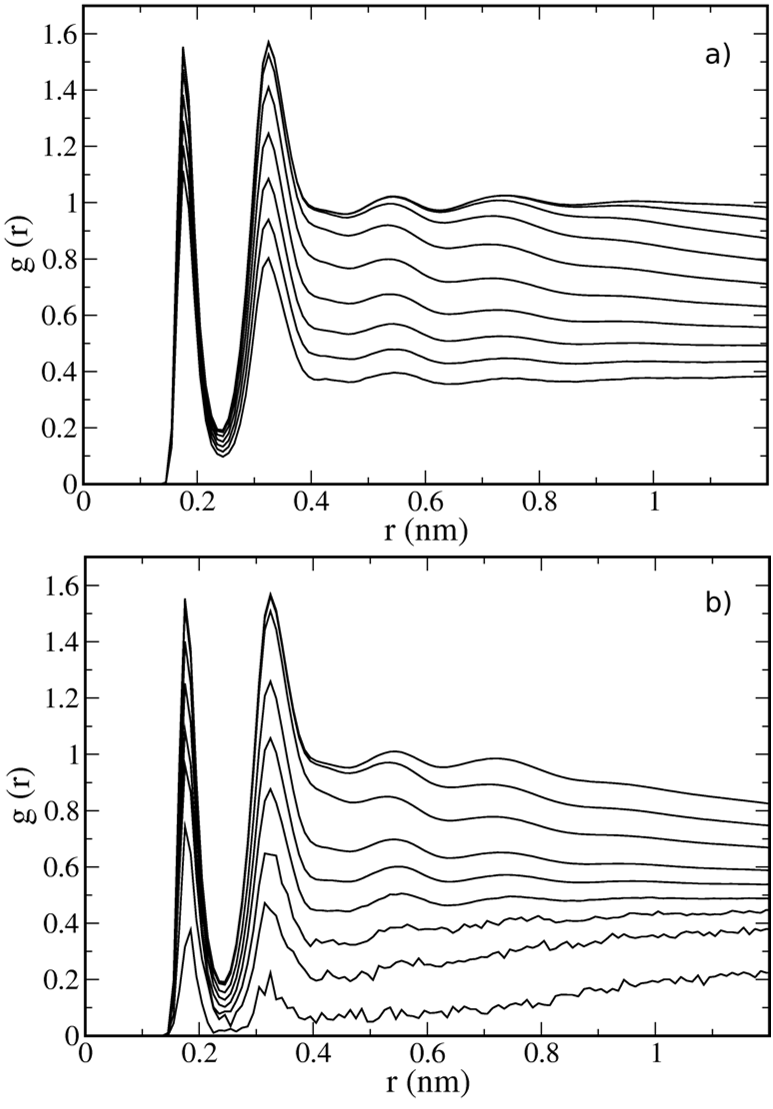
In fact, both interfacial RDFs are similar to their bulk counterparts, but with lower peak intensities and lower limiting values, due to the density depletion at the interface.
as the authors move towards the bulk regions, the diffusion coefficients of both water and nitrobenzene tend toward case i), even though the perpendicular component does not reach the precise bulk value.
These authors have observed that only water molecules at the interface align parallel to the interfacial plane, but that this orientation is correlated with the local curvature of the interface, such that molecules belonging to extrusions of the surface (with positive curvature) align perpendicularly to the interface, with one hydrogen pointing towards the vapor.
Figure 63.2 Interfacial Tension and WidthPerhaps the most important macroscopic property defining an interfacial system is theinterfacial tension.
The square root of the variance of a profile described by equation (10) is no longer equal to we, but is given instead by:( ) eo wAw += 1 (11)Figure 6 shows the result of fitting equation (10) to the intrinsic profiles.