




read more








hemicelluloses and lignin are the three main components of lignocellulosic biomass linked into a complex matrix highly resistant to chemical and biological conversion.
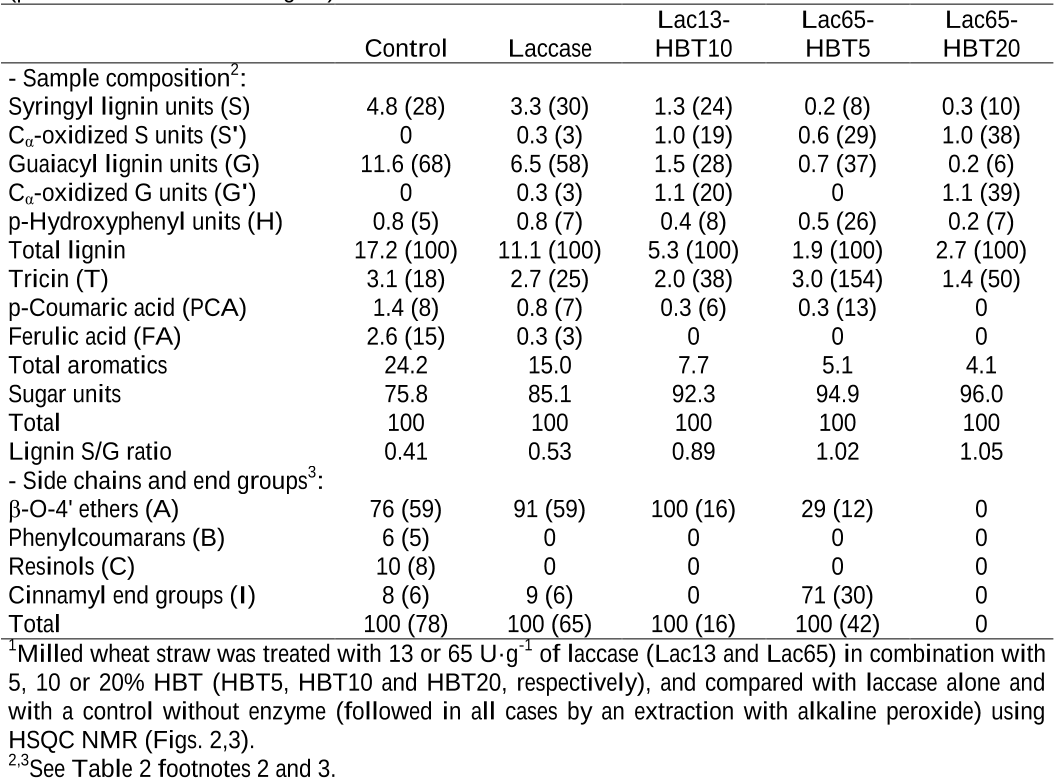
The lignin modification observed in the pretreatments with laccase alone could be due to the action of laccase catalyzing the oxidation of the phenolic moiety (less than 20%) of wheat straw lignin since laccase alone is not able to catalyze the oxidation of non-phenolic lignin units.
Lignin has been shown to have a detrimental effect on the hydrolysis of biomass because it physically hinder the access of cellulases and also binds them reducing activity [5].
The direct action of laccases on lignin is, in principle, restricted to the phenolic units that only represent a small percentage of the total polymer [8], a fact that limits their biotechnological application.
Wheat straw can be delignified by a basidiomycete laccase in the presence of HBT, directly on the ground lignocellulosic material (i.e. without a previous chemical pretreatment) attaining a lignin removal up to 37%.
The sugar degradation and generation of inhibitory compounds during steam explosion that affect the hydrolysis can explain the lower saccharification yields attained in steam-exploded wheat straw.
The novelty of the pretreatment described here, based on the use of a fungal laccase from the basidiomycete Pycnoporus cinnabarinus [24] in combination with HBT as mediator [25], is that it was applied directly in the ground wheat straw feedstock (without a previous chemical pretreatment).
The 13 C1 H correlation signals from the aromatic region of the spectrum were used to estimate the content of lignin, p-coumaric acid, ferulic acid and tricin (compared with the amorphous polysaccharide content, estimated from the anomeric xylose and glucose signals), and the lignin composition in terms of G, S and oxidized S (S') units.
when the latter pretreatment was applied to eucalypt wood, higher delignification degrees (up to 48%) were attained [19,37,38], showing that some woody feedstocks can be competitive for bioethanol production.
From the integrals of the above signals an S/G ratio around 0.4, and a large predominance of β-O4' ether linkages, together with some phenylcoumarans and resinols, were estimated for lignin in wheat straw feedstock, in agreement with previous studies [32].
Other less prominent signals corresponding to phenylcoumaran (B) and resinol (C) substructures were also observed in the HSQC spectrum.
The main signals in the aromatic/unsaturated region of the HSQC spectrum (Fig. 2d) correspond to the benzenic rings and unsaturated side chains of H, G and S lignin units, p-coumaric acid (PCA) and ferulic acid (FA).