
theme are found via the replacement or displacement of one
N atom, thus providing carbenes derived from pyrazolium,
isothiazolium, and even quinolinium salts that contain a
stabilizing heteroatom in a remote position (G-J in Figure
1). Recently, carbenes such as K, which are comprised of
only one heteroatom and lack delocalization through the
heterocycle, have been discovered as versatile ligands, thus
constituting another important class of carbenes with low
heteroatom stabilization. Both the synthesis of the organo-
metallic complexes of these ligands as well as the (catalytic)
properties of the coordinated metal centers generally show
distinct differences, compared to the more classical NHC
complexes, such as C2-metallated imidazolylidenes. This
review intends to describe such differences and highlights
the chemical peculiarities of these types of N-heterocyclic
carbene complexes. It introduces, in a qualitative manner,
the synthetic routes that have been established for the
preparation of such complexes, covering the literature from
the very beginning of activities in this area up to 2008. While
specialized reviews on some aspects of the present topic have
recently appeared,
7
a comprehensive overview of the subject
has not been available thus far. Rather than just being
descriptive, the present account is mainly directed toward
the impact of these still unusual metal-carbene bonding
modes on the electronic properties and on the new catalytic
applications that have been realized by employing such new
carbene complexes. As a consequence of our focus on
complexes with less-stabilized heterocyclic ligands, systems
comprising acyclic carbenes have not been included, and the
interested reader is, instead, referred to the pioneering and
1. Introduction
The organometallic chemistry of N-heterocyclic carbenes
(NHCs) has experienced explosive development during the
last few years, and the topic remains the main focus of many
outstanding research programs.
1
The ongoing popularity of
this research area is certainly due to the development of
extremely active catalyst systems comprising such carbene
ligands. This is perhaps most clearly illustrated by the
second-generation olefin metathesis catalysts developed by
Grubbs and Nolan,
2
or by the cross-coupling catalysts
introduced by Organ and currently commercialized by
Aldrich.
3
The potential of NHCs as ligands for transition metals has
been pioneered, in particular, by the independent work of
O
¨
fele and Wanzlick, and, later, also by Lappert and Stone
in the 1960s and early 1970s.
4
Despite the considerable
progress achieved by these groups, the topic did not attract
widespread attention until Arduengo reported on the isolation
and stability of free N-heterocyclic carbenes.
5
This discovery
marked a watershed in carbene complex chemistry, and these
ligands became available from convenient and inexpensive
precursors such as imidazolium salts. A key factor in the
remarkable stability of Arduengo-type free carbenes lies in
the almost-excessive heteroatom stabilization, because of the
presence of two heteroatoms, at least one of which is
typically a nitrogen in a position R to the carbene carbon (A
in Figure 1).
5
The chemistrysand, specifically, the coordina-
tion behaviorsof these “classical” heterocyclic carbenes has
been reviewed extensively: monographs as well as special
issues have dwelled on this topic.
1
Rather dormant in the beginning of the new millennium,
the concept of heterocyclic carbene ligands that are not
stabilized by two adjacent heteroatoms, as in Arduengo-type
carbenes, and also not necessarily with heteroatoms placed
in a position R to the carbene carbon was revived by a
serendipitous discovery of C4 bonding in imidazolylidenes.
6
The large class of heterocyclic carbenes that can be grouped
together under the title of this review include, in particular,
imidazolium-derived ligands that bind the metal via the C4
or C5 carbon (B and C in Figure 1) as well as the
pyridylidene family with only one heteroatom present in the
heterocyclic skeleton (D-F in Figure 1). Variations on this
Beyond Conventional N-Heterocyclic Carbenes: Abnormal, Remote, and Other
Classes of NHC Ligands with Reduced Heteroatom Stabilization
Oliver Schuster,*
,†
Liangru Yang,
‡
Helgard G. Raubenheimer,
†
and Martin Albrecht*
,‡
Department of Chemistry, University of Stellenbosch, Private Bag X1, 7602 Matieland, Stellenbosch, South Africa, and Department of Chemistry,
University of Fribourg, Ch. du Musée 9, CH-1700 Fribourg, Switzerland
Published in "Chemical Review 109(8): 3445–3478, 2009"
which should be cited to refer to this work.
http://doc.rero.ch
1

ongoing work of Bertrand and co-workers.
8,9
Similarly,
heteroatom-free cyclic carbenes are not further detailed
here.
10
In the literature, different terms have been coined to
describe the bonding of such less-stabilized carbenes to metal
fragments. For example, terms such as “wrong way”,
“abnormal”, “unusual”, or “nonclassical” have been used to
describe C4/C5-bound imidazolylidenes (B). Throughout this
review, we refer to “abnormal” carbenes as those NHC
ligands for which a canonical valence bond representation
requires the introduction of additional formal charges on
some nuclei (e.g., B, C, E,orI in Figure 1). The term
“remote” carbene indicates that no heteroatom is located in
a position R to the carbene carbon (e.g., E, F, H, I in Figure
1); it may be possible to write uncharged contributing
resonance structures for the free ligand.
11
A final preliminary remark concerns the controversial
classification of all these ligands as “carbenes”. While this
classification implies that the ligand is a neutral donor, in
all instances, a zwitterionic canonical representation consist-
ing of a carbanionic and a cationic iminium center may be
similarly appropriate and even necessary. When bonded, this
negative charge is obviously transferred to the metal in one
canonical form. Clearly, the borderline between the two
limiting representations is continuous, and the issue of
whether a ligand is, in reality, a carbene or not may become
semantic. In the case of the C2-bound imidazolylidenes,
experimental and theoretical studies are in agreement with
a relatively small π-contribution to the M-C bond only (M
) electron-rich metal center),
12-14
and, hence, the M -C
interaction is typically represented by a single bond. How-
ever, detailed studies involving less-stabilized N-heterocyclic
carbenes are still rare. Often, crystallographic and NMR
spectroscopic arguments have been put forward to support
one resonance form or the other. Despite the fact that the
metal-carbon bonds in Fischer carbenes and in N-hetero-
cyclic carbenes are very much related, different means of
representation have evolved in the literature. In this review,
single bonds are used to represent M-C
carbene
interactions,
which is consistent with the accepted representations of
conventional NHC-metal bonds and even other metal-ligand
bonds that are known to comprise significant π-character
(e.g., the M-CO bond in carbonyl complexes). Classical
Fischer-type carbene complexes are written with an M)C
double bond, in agreement with a different convention
developed in the 1960s. A more complete discussion of these
Table 1. Available Methods for NHC Metallation
metallation method ligand system
via free carbene • 2-imidazolylidenes and related ligands
a
• 4-imidazolylidenes (via 2-imidazolylidene rearrangement)
• cyclic (amino)(alkyl)carbenes (CAACs)
and (amino)(ylide)carbenes (AYCs)
C-H bond activation • 2-imidazolylidenes and related ligands
a
• 4-imidazolylidenes
• 4-triazolylidenes
• 3-pyrazolylidenes
• 2-, 3-, 4-pyridylidenes
• CAACs and AYCs
C-E bond activation • 2-imidazolylidenes (E ) CH
3
: activation with Ag
I
; E ) CO
2
-
:
activation with d
8
metals; CdC activation of enetetramines)
• 2-pyridylidenes (E ) PR
2
: activation with Pd
II
)
C-X oxidative addition • 2-imidazolylidenes and related ligands
a
• 4-imidazolylidenes
• 3- and 4-pyrazolylidenes
• 2-, 3-, 4-pyridylidenes
• CAACs
transmetallation • 2-imidazolylidenes (predominantly from Ag complexes)
• 3-pyrazolylidenes (from Ag, Cr)
• 4-imidazolylidenes
• 4-triazolylidenes
• 2-pyridylidenes (from Cr)
heteroatom alkylation • 3-pyrazolylidenes
• 2-, 3-, 4-pyridylidenes
cycloaddition to Fischer carbenes • 2-imidazolylidenes and related ligands
a
• 3-pyrazolylidene
• 2- and 4-pyridylidene
• expanded ring NHCs
a
Includes NHCs with two different stabilizing heteroatoms in a position R to the carbene.
Figure 1. N-heterocyclic carbenes, including the “classical” NHC
representative (A) and representatives of subclasses comprising
reduced heteroatom stabilization (B-K); all are shown in their
carbene form.
http://doc.rero.ch
2

considerations is provided in Section 3, after synthetic
strategies have been introduced. The review concludes with
applications of such carbene complexes in catalysis.
2. Methods of Ligand Complexation
A variety of different methods have been established for
the complexation of less-heteroatom-stabilized NHC ligands.
Some of these methods are very similar to those yielding
normal C2-bound imidazolylidene complexes, while others
are unique to a particular subclass of NHC ligands. The
different methods of NHC ligand complexation are compiled
in Table 1. Further details are provided in this section, which
has been organized according to the different ligand systems
involved, rather than according to the methods used.
2.1. Complexes with C4-Bound Imidazolylidenes
2.1.1. C-H Bond Activation of Unsubstituted
2H-Imidazolium Salts
Crabtree and co-workers
15
were the first to observe
abnormal C4 metallation of imidazolium salts a few years
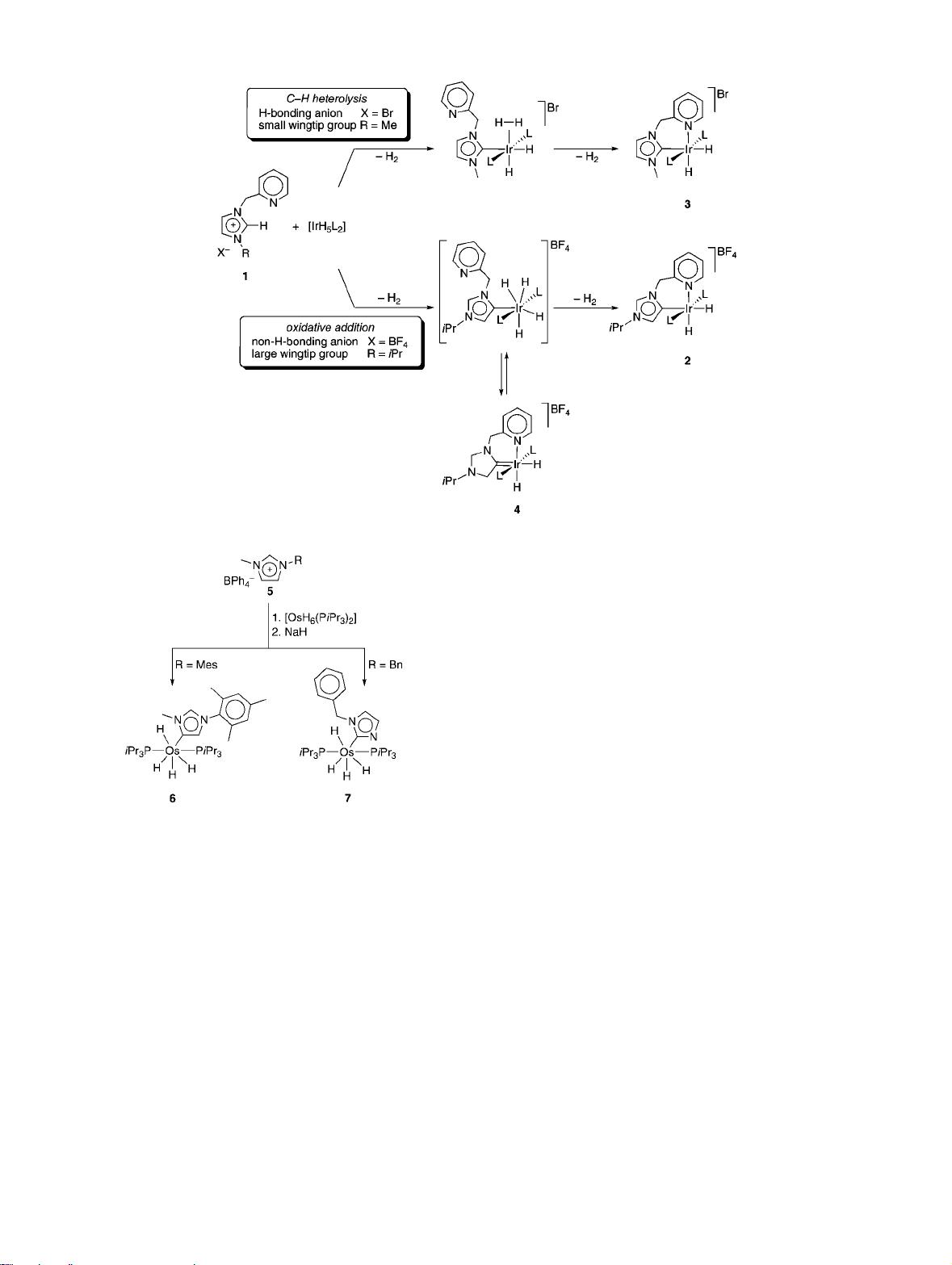
ago (Figure 2). The reaction of pyridine-functionalized
imidazolium salt 1 with the iridium polyhydride IrH
5
(PPh
3
)
2
also afforded the iridium (III) complex 2, which is comprised
of a carbene that is abnormally bound through C4 rather than
C2 (see Scheme 1). The coordination mode was deduced
from NMR spectroscopy and was unambiguously confirmed
by X-ray crystallographic analysis. No interconversion to the
presumably more-stable normal carbene complex 3 was
detected. Hence, product formation seems to be kinetically
controlled. These results indicated, for the first time, that it
may not always be safe to assume C2 bonding when
preparing NHC complexes in situ from imidazolium salts
and a metal precursor.
The activation of the C4-H bond in imidazolium salts
such as 1 is remarkable when considering the acidity
difference between the two types of heterocyclic protons.
The acidity of the proton attached to C2 has been determined
experimentally and by calculation (pK
a
) 24 ( 1).
16
This
value is 9 pK
a
units lower than that calculated for the C4-
bound proton (pK
a
) 33).
17
The difference suggests that
aspects other than the acidity of the protons control the
regioselectivity of metallation.
The selective formation of C4- or C2-bound carbene
complexes with iridium hydrides seems to be dependent on
multiple factors.
18
Calculations suggest that C2 bonding and
C4 bonding proceed via distinctly different reaction pathways
involving either C2-H heterolytic bond cleavage or C4-H
oxidative addition, implicating an iridium(V) species (see
Scheme 2).
19
Such mechanistic proposals were further
supported by experimental data, which demonstrate that
product distributionsand, thus, the site of metallationsis
strongly anion-dependent. Large anions such as BF
4
-
typi-
cally are only weak partners for hydrogen bonding and effect
small changes in charge distribution. Consequently, such
anions favor an oxidative addition pathway, leading to
carbene C4 bonding. In contrast, smaller counterions such
as Br
-
accelerate heterolytic C-H bond cleavage through
hydrogen bonding, thus supporting a proton migration from
the imidazolium moiety to the metal-bound hydride. Ac-
cordingly, such anions preferentially yield C2-bound car-
benes. Time-dependent NMR analysis of the formation of 2
has revealed the intermediate formation of a hydrogenated
imidazolinylidene species 4.
15
This result is consistent with
an oxidative addition pathway that is comprised of an [IrH
4
]
+
species, which may reversibly transfer H
2
from the metal
center to the imidazolylidene heterocycle. Notably, chelation
of the pyridine moiety is not essential and similar selectivities
in C-H bond activation have been observed with simple
imidazolium salts upon reaction with [IrH
5
(PPh
3
)
2
]inthe
presence of pyridine.
20
Recent studies by Esteruelas et al. on the metallation of
imidazolium salts such as 1 with the osmium hydride
precursor [OsH
6
(PiPr
3
)
2
] have confirmed the relevance of the
counteranion for the regioselectivity of metallation.
21
Met-
allation at the C4 position is again favored with large and
unpolarized [BPh
4
]
-
anions, whereas imidazolium bromides
afford, almost exclusively, the C2-metallated carbene. Time-
dependent analysis of carbene formation indicated that kinetic
factors are more relevant for C4 coordination than for C2
coordination. In addition, isomerization of the C4-bound
carbene to its thermodynamically favored C2-bound isomer
has been accomplished under strongly acidic conditions in
the presence of HBF
4
.
The regioselectivity of metallation is further influenced
by the wingtip substituents on the imidazolium salt.
22
A
mesityl substituent promotes C4 bonding to the Os center 6,
while the corresponding benzyl-substituted imidazole gives
the C2-bound carbene complex 7 in high yields (see Scheme
3). The outcome of this reaction can be explained by
invoking steric hindrance between the isopropyl groups of
the phosphines and the imidazolium wingtip groups, which
is more pronounced for mesityl than for the comparatively
flexible benzyl substituent.
A driving force different from counterion effects and
steric discrimination is required to rationalize the selective
C4 metallation of the imidazolium salt 8, which is
comprised of a chelating phosphine wingtip group to give
complex 10 (see Scheme 4).
23
With [Ir(cod)Cl]
2
(where cod
) 1,5-cyclooctadiene), initial phosphine coordination and
formation of 9 has been observed. Subsequent C-H bond
activation occurs exclusively at the C4-position and is
reversible with ethylene-linked bidentate ligands, yet slow
and irreversible with the analogous methylene-bridged
derivative 9a. Base-mediated reductive elimination affords
the corresponding iridium(I) complexes 11. Furthermore,
neither a small wingtip group nor a hard chloride counterion
(not shown) succeeds in promoting C2-H bond activation.
Perhaps the affinity of iridium(I) for olefinic CdC bonds
Figure 2. Metal complexes comprising normal (L) and abnormal
(M) imidazolylidene ligands bound at the C2 and the C4 position,
respectively.
Scheme 1
http://doc.rero.ch
3
might also play a role in the regioselectivity of iridation. In
addition, the constrained bulk of the coordinated phosphine
ligand could increase the steric sensitivity of the Ir
center.
Additives also have a distinct influence on the regiose-
lectivity of imidazolium palladation. Metallation of the
hydrochloride adduct of N,N′-dimesitylimidazol-2-ylidene
(IMes · HCl) with Pd(OAc)
2
, in the presence of Cs
2
CO
3
as a
base, occurs selectively at the C2-position, thus affording
the normal bis(carbene) complex 12 (see Scheme 5).
24
In
the absence of Cs
2
CO
3
, however, the heteroleptic complex
13 is formed. It is comprised of one C2-bound NHC ligand
and one carbene that is bound abnormally at C4 to the
palladium center (see Scheme 5). Interestingly, an X-ray
structure analysis shows that the two different Pd-C bond
lengths are identical within experimental error (Pd-C )
2.019(13) and 2.021(11) Å for the normal and abnormal
carbene, respectively). According to the mechanistic model
used for iridium metallation (Vide supra), the CO
3
2-
anion
may promote heterolysis of the most acidic C-H bond, thus
favoring formation of C2-bound complexes. In the absence
of a base, the C4-H bond is activated, probably by oxidative
addition, to give 13. The trans orientation of the two carbene
ligands seems to play a decisive role for C4 bonding. In
rigidly cis coordinating, chelating bis(carbene) complexes,
exclusive C2 bonding is observed under identical base-free
metallation conditions.
25,26
2.1.2. C-H Bond Activation of C2-Substituted
Imidazolium Salts
Although the previous section illustrates the feasibility of
C4 bonding with 2H-imidazolium salts, which may be
particularly relevant for in situ complex formation, unpro-
tected imidazolium salts are primarily metallated at the C2-
position. A rational route toward C4-bound carbenes there-
fore includes the selective protection of the most acidic C2-
position, e.g., by incorporating alkyl or aryl substitutents.
Thus, oxidative addition of the C4-H bond of the tetra-
alkylated C2-blocked imidazolium salt 14 to zerovalent
Pt(norbornene)
3
, in the presence of equimolar amounts of
the free carbene IMes, yields the platinum hydride complex
15 with the mixed C2- and C5-bound carbenes both attached
to platinum (see Scheme 6).
27
The formation of this complex
has been proposed to occur stepwise. Initial coordination of
the basic IMes provides the necessary electron density at
the central metal to allow for subsequent oxidative addition
of the imidazolium C4-H bond. A similar reaction sequence
may apply to the formation of the abnormal/normal
[Pd(IMes)
2
Cl
2
] complex 13 (Vide supra). When using the
asymmetrically 1,2,3-trialkylated imidazolium precursor 16,
a mixture of C4- and C5-bound isomers 17a and 17b is
formed in a 3:1 ratio. This product distribution might reflect
a moderate steric preference in the transition state of the
oxidative addition.
Complexes 17 are unstable in the presence of certain
alkenes, such as styrene, and undergo reductive elimination.
The C4-bound carbene is significantly more prone to
reductive elimination than the C2-bound IMes ligand, leading
to the exclusive formation of the imidazolium salt 16 and
Scheme 2
Scheme 3
http://doc.rero.ch
4

the Pt
0
complex, Pt(IMes)(diolefin). No products resulting
from reductive elimination of the normal C2-bound carbene
nor from alkene insertion into the Pt-H bond are observed.
Both, electronic and steric reasons may account for the
observed reaction outcome, and further investigations are
clearly desirable.
C4-bound carbene metal complexes can also be made by
transmetallation from the corresponding silver complexes.
Precursor Ag-NHC complexes are typically generated from
silver oxide (Ag
2
O) and imidazolium salts.
28
To achieve
selective metallation, it is necessary to protect both the C2-
and C5-positions. For example, the disubstituted imidazolium
salt 18 undergoes clean deprotonation in the presence of
Ag
2
O.
20
Subsequent transmetallation of the presumably
formed silver complex with [Ir(cod)Cl]
2
yields the Ir
+
complex 19a, and after the exchange of spectator ligands
(cod for CO) complex 19b (see Scheme 7). IR spectroscopy
of this dicarbonyl complex allows for an estimation of the
electron-donating ability of such C4-bound carbenes. From
the observed stretching frequencies (ν
CO
) 2045, 1961 cm
-1
),
a Tolman electronic parameter (TEP)
29
of ν ) 2039 cm
-1
has been estimated. This value is considerably lower than
for analogous C2-bound carbenes (ν ≈ 2050 cm
-1
) or basic
phosphines (cf. PCy
3
, ν ) 2056 cm
-1
). Hence, such C4-
bound carbenes are among the best neutral donors known.
Complex 19a has been demonstrated to be a useful metal
precursor for transmetallation. In the presence of a Ag-
triazolylidene, swift formation of the normal/abnormal bis-
(carbene) complex 20 is observed.
30
Complexes such as 20
generally exist as multiple diastereoisomers, since rotation
about the Ir-C
carbene
bonds is hampered by the two cis-
coordinated carbene ligands. Initial attempts to separate the
diastereoisomers of 20 by recrystallization have been unsuc-
cessful; yet, this may become an attractive methodology for
application in asymmetric catalysis.
Notably, the formation of stable abnormal silver carbene
complexes for transmetallation is often limited to imidazo-
lium salts with aryl substituents at the C2-position, because
primary or secondary alkyl groups have been found to be
unreliable protecting groups.
31
Reaction of Ag
2
O with
2-methylated or 2-benzylated imidazolium salts 21 initiates
an unexpected C-C bond activation process, thus yielding
the normal Ag-carbene complex 22 (see Scheme 8). A
detailed analysis of the course of reaction reveals that Ag
2
O
is gradually oxidizing the carbon that is attached to C2 to
yield acyl imidazolium salts and metallic silver. In the
presence of water that is formed during this redox reaction,
acyl functionalities seem to be good leaving groups and,
hence, promote metallation at the C2 carbon. Consistent with
this mechanistic scheme, the highest yields are obtained when
a large excess of silver salt is used. A similar oxidation is
effectively suppressed when a quaternary carbon (e.g. a
phenyl group) is attached to C2.
A transmetallation protocol has been applied for the
synthesis of a series of complexes 24 that are comprised of
abnormally bound imidazolylidene-derived ligands (see
Scheme 4
Scheme 5
Scheme 6
http://doc.rero.ch
5





