
BRIEF REVIEW
www.jasn.org
Diagnosis and Treatment of Hyponatremia: Compilation
of the Guidelines
Ewout J. Hoorn and Robert Zietse
Department of Internal Medicine, Division of Nephrology and Transplantation, Erasmus Medical Center, Rotterdam, The
Netherlands
ABSTRACT
Hyponatremia is a common water balance disorder that often poses a diagnostic or
therapeutic challenge. Therefore, guidelines were developed by professional orga-
nizations, one from within the United States (201 3) and one from within Europe
(2014). This review discusses the diagnosis and treatment of hypona tremi a, com-
paring the two g uideli nes and highlighting r ecent developments. Diagnostically, the
initial step is to differentiate hypotonic from nonhypotonic hyponatremia. Hypo-
tonic hyponatremia is further differentiated on the basis of urine osmolality, urine
sodium level, and vo lume st atus. Recently identified parameters, including frac tional
uric acid excretion and p lasma copeptin concentration, may further impro ve the
diagnostic approach. The treatment for hyponatremiaischosenonthebasisof
duration and symptoms. For acute or severely symptomat ic hyponatremia, both
guidelines adopted the approa ch of giving a bolus of hypertonic saline. Although
fluid restriction remains the first-line treatment for most forms of chronic hypona-
tremi a, therapy to increase renal fr ee water excretion is often necessary. Vasopres-
sin recep tor antagonists , urea, and loop d iuretics serve this purpose, but received
different recommendations in the two guidelines. Such discrepancies may relate to
different interp retations o f the limited evidence or differences in guidel ine meth-
odology. Nevertheless, the development of guidelines has been important in ad -
vancing this evolving field.
J Am Soc Nephrol 28: 1340–1349, 2017. doi: https://doi.org /10.168 1/ASN.201610 1139
Hyponatremia (serum sodium [S
Na
]
,136 mmol/L) is a common water bal-
ance diso rder that often poses a diagnos-
tic or therapeutic challenge.
1
This may
explain why management of hyponatre-
mia is still suboptimal, as also recently
illustra ted by a hyponat remia registry.
2
Hyponatremia is not a disease but
rather a pathophysiologic process ind i-
cating disturbed water homeostasis.
3
Therefore, hypo natremia should be fur-
ther classified in order to provide direc-
tions for diagnosis and treatment (Tabl e
1). These classifications illustrate that
hyponatremia is a ver y heterogeneous
disorder. This has complicated clinical
studies, because “the” patient with hypo-
natremia does not exi st. Instead, the un-
derlying disease that is complicated by
hyp onatremia usually characterizes pa-
tients with hy pona tremia.
4,5
The most
common causes of hyponatremia are
the syndrome of inappropriate antidiu-
resis (SIAD), diuretic use, polydipsia,
adrenal insufficiency, hypovolemia,
heart f ailure, and liver cirrhosis (the lat-
ter two are often collectively referred to
as “hypervolemic hyponatremia”). Al-
though recent years have seen several
developments in the diagnosis and treat-
ment of hyponatremia, the evidence base
is still limited. To capture the current
approach to hyponatremia, two sets of
guidelines have been developed, one by
professional organizations from within
the United States (“United States guide-
line”) and one from within Europe (“Eu-
ropean guideline,” in which the authors
of this review participated).
6–9
The pro-
fessional organizations involved in the
United States guideline were Tufts Uni-
versi ty Office of Continuing Education
and In 2 MedEd; the initiative was
also supported by an unrestricted edu-
cational grant from Otsuka A merica
Pharmaceutical.
9
The professional orga-
nizations involved in the European
guideline w ere the European Renal As-
sociation–European D ialysis and Tra ns-
plantation Association, the European
Society o f Endocrinolog y, and the Euro-
pean Societ y of Intensive Care Medi-
cine.
6–8
The United States guideline
refrained from using a quality-of-evidence
scoring system due to the limited evi-
dence. Instead, the guideline was on
the b asis of expert panel recommenda-
tions, which relied on a critical evalua-
tion of relevant literature by the panel
members. The European guideline
did perform systematic reviews of the
available evidence using the Grading of
Published online ahead of print. Publication date
available at www.jasn.org.
Correspondence: Dr. Ewout J. Hoorn, Erasmus
Medical Center, Internal Medicine – Nephrology,
Room D-438, PO Box 2040, 3000 CA, Rotterdam,
The Netherlands. Email: e.j. hoorn@erasmusmc.nl
Copyright © 2017 by the American Society of
Nephrology
1340 ISSN : 1046-6673/2805-1340 JAmSocNephrol28: 1340–1349, 2017

Recommendations Assessment Develop-
ment and Evaluation s coring system.
Both guideline committees were interdis-
ciplinary, and the European guideline was
endorsed by the European societies of ne-
phrology, endocrinology, and intensive
care.
6–8
This brief review will compare
the two guidelines to discuss the diagnosis
and treatment of hyponatremia, while
also hig hlig hting recent developments.
Because of the breadth of both guidelines,
this review will focus on the salient fea-
tures. To place both guidelines in perspec-
tive we will integrate in our discussion the
pertinent comments published after their
release.
10–13
DIFFERENTIAL DIAGNOSIS OF
HYPONATREMIA
Although th e United States guideline did
not present a diagnostic al gorithm, the
classifications of hyponatremia on the
basis of tonicity and volume status
were discussed.
9
The initial differe ntia-
tion in hypoton ic an d nonhy potonic
hy ponatremia is important, because
management is different.
14
No nhypo-
toni c hy ponatremia is usually caused by
hyperglycemia, but may also be caused
by the administration of mannitol or
hypertonic radiocontrast.
7
In these set-
tings, manag ement is usually conserva-
tive, although a decrease in ex tracellular
toni city may occur during treatment.
15
Nonhypotoni c hyponatremia can also be
caused by pseudohyponatremia, a labora-
tory artifact that may occur with high con-
centrations of triglycerides, cholesterol,
or protein.
16
The United States guide-
line subsequently div ided hyp otonic
hyponatremia into hypovolemic, euvo-
lemic, and hy pervolemic hyponatre-
mia.
9
Although this represents the
most traditional and commonly used
approach to hypotonic hyponatremia,
it deserves scrutiny. Hypovolemic and
euvolemic hyponatremia are notori-
ously d ifficult to differentiate on the ba-
sis of physical examination,
17
whereas
hypervolemic hyponatremia is usu ally
clinically obvious (presence of edema
or ascites). Two studies that a nalyzed
the diagnostic performance of the clin-
ical assessment of volume status in pa-
tients with hyponat remia reported low
sensitivity (50%–80%) and specificit y
(30%–50%) .
18,19
Previously, we showed
that clinicians of ten misclassify hypona-
tremia when using algorithms that start
with clinical assessment of volume st a-
tus.
20
Similarly, physicians in training
had a better diag nostic performance
than senior physicians when using an
algorithm in which urin e osmolalit y
(U
Osm
) and urine sodium (U
Na
)con-
centration are prioritized over assess-
ment of volume status.
21
Because the
kidneys will respond to hypovolemia
or a low effective ar ter ial blood volume
with sodium retention, U
Na
,30 mmol/L
can be used to identify bot h hypovole-
mic and hypervolemic hyponatremia.
Three caveats, however, should be em-
phasized: (1)U
Na
will also be low in pa-
tients consuming a low sodium diet
(rareinthewesternpopulations),(2)
the (recent) use o f diuretics will increase
U
Na
,and(3) p atients with CKD may be
less able to reabsorb sodium.
7,22
In ad-
dition, advanced CKD usually impairs
water excretion, complicating the eval -
uation of the role of vasopressin in water
balance.
23
These c onsiderations prompted
the European guideline committee
to propose an algorithm that prioritizes
U
Osm
and U
Na
over volume status (Fig-
ure 1). It also incorporates the limita-
tions of U
Na
. In addition, it recommends
early identification of acute o r sy mp-
tomatic hyponatremia to identify pa-
tients in whom immediate treatment is
indicated. Two additional d iagnostic
tests for hyponatremia mer it discussion,
including a trial of volume expansion
and the fractional uric acid excretion
(FE
UA
). A trial of volume expansion
with isotonic saline can be use d to diag-
nose hypovolemic hyponatremia.
18
Although a rise in S
Na
in response to iso-
tonic saline would be consistent with
hy povolemic hyponatremia, another
possibility would be that the stimulus
for vasopressin release in a patient w ith
SIAD abated. Such stimuli are often
nonspecific and transient, including
pain or nausea.
14,24
In addition, S
Na
has been shown to improve upon saline
infusion in patients with SIAD w ith
U
Osm
,500 mOsm/kg .
25
Conversely,
isotonic saline may sometimes worsen
hyponatremia, a phenomenon called
“desalination.”
26
In response to the
United States guideline, Gross raised
the issue of how to deal w ith mixed
forms of hyponatremia, for example
SIAD and hypovolemia.
10
Indeed, we
previously showed that patient s ofte n
have two to three possible causes for
Table 1. Classifications of hyponatremia
Classification Criteria
Limitations of
Clinical Utility
Moderate (125–129 mmol/L)
versus severe/profound
a
(,125 mmol/L)
Absolute S
Na
concentration Symptoms do not always
correlate with degree of hyponatremia
Acute versus chronic Time of developm ent (cutoff 48 h) Time of development not always known
Symptomatic versus asymptomatic Presence of symptoms Many symptoms aspecific; chronic
hyponatremia may be symptomatic
Hypotonic, isotonic, or hypertonic Measured serum osmolality Ineffective osmoles (e.g ., urea, ethanol)
are also measured
Hypovolemic, euvolemic, hypervolemic Clinical assessment of
volume status
Clinical assessment of volume status
has low sensitivity and specificity
a
S
Na
,125 mmol/L is defined as “severe hyponatremia” by the United St ates guideline, and as “profound hyponatremia” by the European guideline.
7,9
J Am Soc Nephrol 28: 1340–1349, 2017 Hyponatremia 1341
www.jasn.org
BRIEF REVIEW
hyponatremia (although it was unclear
if and to which extent each cause con-
tributed).
27
In addition to a trial of vol-
ume repletion, an alternative approach
to mixed pathogenesis would be to com-
bine hypertonic saline with desmopres-
sin.
28,29
Although the literature on this
approach is limited, it offers a rational
approach to prevent a rapid r ise in S
Na
that may occur once hypovolemia h as
been corrected. Fenske et al. found that
FE
UA
.12% had the hig hest sensitivit y
and specificit y to diag nose SIAD with
or w ithout diuretic use.
30
This study is
of interest because it formally tested the
diagnostic performance of several pa-
rameters using receiver operating
curves. More recently, a larger study
confirmed that FE
UA
.12% had the
best sensitivity and specificity for
SIAD.
31
In absolute terms, however, the
performance of FE
UA
was still moderate,
and U
Na
.30 mmol/L and FE
Urea
.55%
had better sensitivity and specificity for
SIAD, respectively. We frequently ana-
lyze FE
UA
in patients with hyponatremia,
but mainly use it as supporting informa-
tion. FE
UA
is high in both SIAD and
cerebral salt wasting, but normalizes in
SIAD only during treatment.
32
Of note,
however, is that even in neurosurgical
patients with hyponatremia, cerebral
salt wasting is rare and has remained
an enigmatic and not widely accepted
clinical entity.
33,34
VASOPRESSIN
Arginine vasopressin (the antidiuretic
hormone) plays a central ro le in the path-
ogenesis of hyponatremia. In one study,
nonosmotic secretion of vasopressin was
detected in 97% of patients with hypo-
natremia.
35
Because hypotonicity nor-
mally suppresses vasopressin, the
reasons for nonosmotic vasopressin
release should be considered.
36
“Appro-
priate” vas opressin release is due to hy-
povolemia or low effective arterial blood
volume, both of which activate barore-
ceptors to cause vasopressin release.
Although one might expect thiazide-
induced hyponatremia to be due to
hypovolemia secondar y to saliuresis,
this is not the case.
37
Instead, the path-
ogenesis appears to be a combinati on of
polydipsia and impaired urea-mediated
water excretion.
37,38
“Inappropriate” va-
sopressin release is usually caused by the
effect of an underl ying disease or drugs
on central osmoreceptors; alternatively,
vaso pressin can be produced ectopically
(e.g., in smal l cell lung cancer or olfac-
tory neuroblastoma).
3,39,40
In additi on,
hy pocortisolism increases vasopressin
release, because cort icotropin -releasing
hormone normally suppresses vasopres-
sin.
41
Although rare, secondary and even
primar y adrenal insufficiency may
mimic SIAD and can be missed w ithout
appropriate testing .
42–44
Although the
kidney usu ally limits the degree of hypo-
natremia in SIAD (“ vasopressin es-
cape”
45
), it can also cause antidiuresis
independent of vasopressin.
46,47
Aspe-
cific example is gain-of-function muta-
tions of the vasopres sin type 2 receptor
causing hereditary hyponatremia
(“nephrogenic SIAD”).
48
Despite the
pathogen etic role of vasopressin in hy-
ponatremia, pl asma vasopressin is rarely
measured in clinical practice. This has
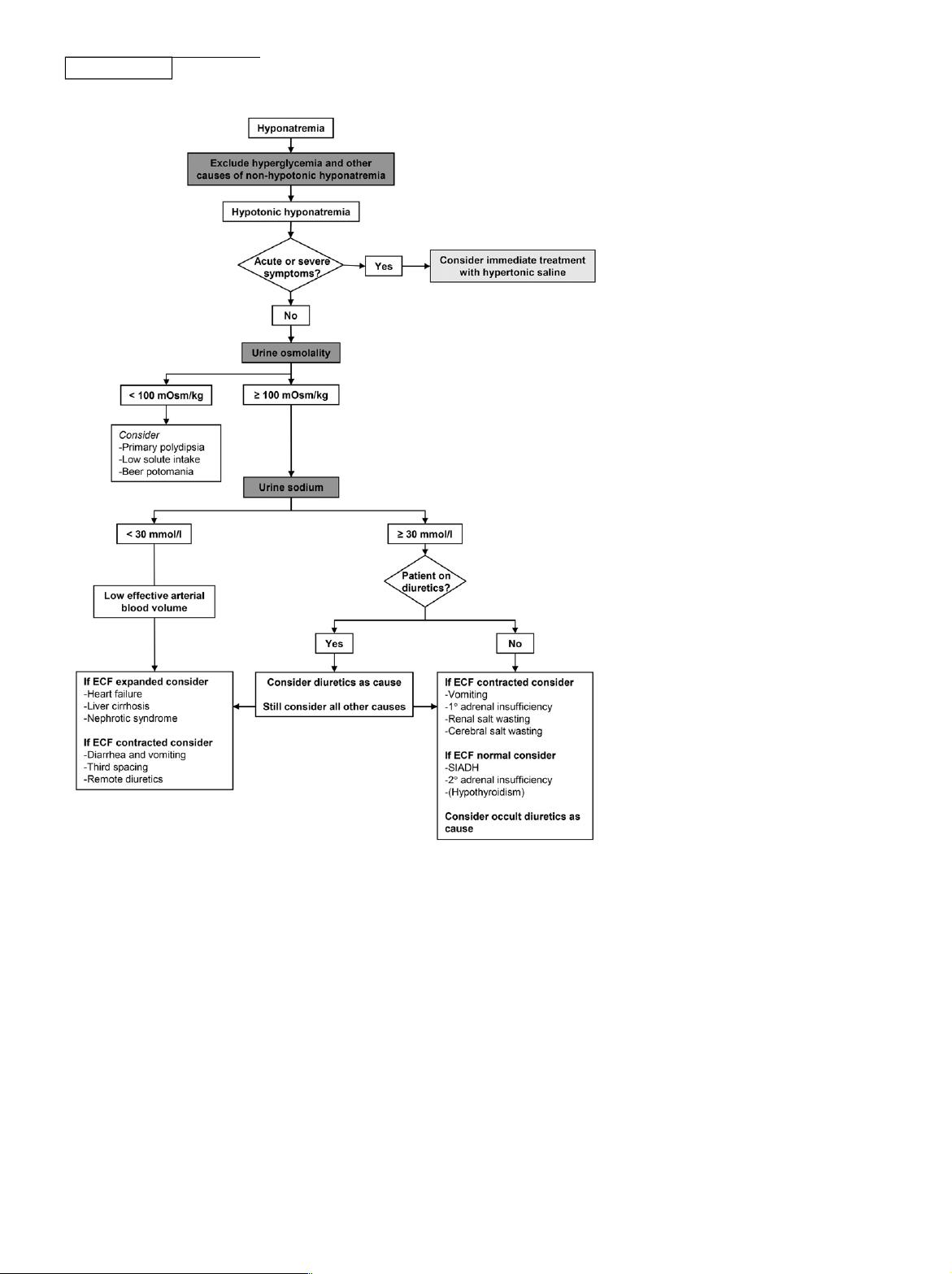
Figure 1. Diagnos tic algorithm for hyponatremia. Based on the European guideline.
7
ECF,
extracellu lar fluid.
1342 Journal of the A merican Society of Nephrology J Am Soc Nephrol 28: 1340–1349, 2017
BRIEF REVIEW www.jasn.org

two reasons. First, U
Osm
accurately re-
flects vasopressin activity, and, therefore,
this more readily available parameter can
be used instead. Second, vasopressin is
difficult to measure reliably in nonexpert
laboratories, because it binds to platelets,
it is unstable in isolated plasma, and com-
mercial assays are not very sensitive for
low concentrations.
49
These limitations,
however, have largely been resolved
by the development of an assay for
copeptin.
50
COPEPTIN
Enzym atic cle avage o f the vasopressin
prohormone produces not only vaso-
pressin, but als o neurophysin and copep-
tin (also called C-t erminal proarginine
vasopressin).
51
Because copeptin is
more stable, it can be measured more
easily. Copeptin can the refore be used
as a surrogate marker for vasopress in.
Although both guidelines only briefly
discuss copeptin, emerging data
justify a brief discussion on the diagnos-
tic utility of this novel marker. Fenske
et al. found that plasma copeptin levels
were higher in patients with hypo- or
hypervolemic hyponatremia than in pa-
tients w ith SIAD.
52
This was demon-
strated p reviously
35
and likely reflects
an “osmoreceptor gain,” the phen ome-
non in which angiotensin II amplifies
vasopressin release in the context of a
low effective arterial blood vol ume.
53,54
Because hypovolemic hyponatremia is
characterized by h ig h plasma copeptin
and low U
Na
, the plasma copeptin to
U
Na
ratio may be especially u seful to dif-
ferentiate it from SIAD. Although the
study by Fenske et al. did indeed dem-
onstrate this,
52
the specificity of co pep-
tin/U
Na
for SIAD in a more recent and
larger study was less high.
31
An interest-
ing approach was the use of plasma
copeptin to differentiate SIAD sub-
types.
55
Using hypertonic saline, SIAD
subty pes were defined on the basis of
their relationship between serum os-
molality and plasma copeptin (Figure
2). As expected, low plasma copeptin
levels are diagnosti c f or hyponatremia
due to polydipsia.
31,52
Arguably, the
need for a novel diagnostic marker fo r
this cause of hyponatremia is limited, as
it is usually obvious from the clinical
setting and the low U
Osm
.Inaddition
to plasma copeptin, two additional cir-
culating markers were recently evalu-
ated in patients with hyponatremia,
including apelin and midregional
proatrial natriuretic peptide (MR-
proANP).
56,57
Physiologically, apelin
and va sopressin are regulated in oppo-
site directions by volemic and osmotic
stimuli.
56
Apelin not only inhibits vaso-
pressin release centrally, but also coun-
teracts the antidiuretic effect in the
kidney.
58
However, in pat ient s wit h hy-
ponatremia due to SIAD o r heart failure,
plasma apelin was insufficiently sup-
pressed, possibly contributing to anti-
diuresis in these settings.
56
Similar to
plasma copeptin, MR-proANP levels
were higher in patients with hypovole-
mic or hyper volemic hyponatremia
than in patients w ith SIAD (although
these levels were still hig her than in
healthy subjects).
57
High MR-proANP
in hypovolem ic hyp onatremia is coun-
terintuitive, but may be explained by
lower GFR secondary to volume deple-
tion.
59
Although plasma copeptin, ape-
lin, and MR-proANP increase insig ht
into the pathophysiology of hyponatre-
mia,thetruediagnosticpotential
of these parameters remains to be de-
termined. In addition, one single pa-
rameter is unlikely to achieve optimal
Figure 2. Copeptin-based classification of five subtypes of the syndrome of in-
appropriate antidiuresis (SIAD). The shaded gray area a nd the black dashed line show
the norma l physiolo gic rela tionship between serum o smolality and plasma copeptin (as
surrogate marker for vasopressin). In SIAD type B this relationship is intact, but the
osmotic thres hold for vasopressin release has decreased. In SIAD types A and C va-
sopressin release is no longer regulated by serum osmolality. In SIAD type D plasma
copeptinlevelsare undetectable.InSIAD typeE thenormalrelationshipbetweenserum
osmolality and copeptin has reversed. This phenomenon has been coined “barostat
reset,” as it may indicate increased sensitivity of baroreceptors to increased vaso-
pressin r elease. Percentages indicate ho w often each subtype was present in one study
of 50 patients. Data on the basis of Fenske et al.
55
and figure modified from Fenske
et al.
116
with permission.
J Am Soc Nephrol 28: 1340–1349, 2017 Hyponatremia 1343
www.jasn.org
BRIEF REVIEW

discriminator y power. A relevant ques-
tion i s whether a combination o f diag-
nostic parameters might improve
management.
GENERAL APPROACH TO
TREATMENT
A cutoff of 48 hours is usually used to
differentiate acute from chronic hypo-
natremia (Table 1).
7
This classification
is useful because acute and chronic hy-
ponatremia may be complicated by
different neurologic conditions. Acute
hyponatremia can cause cerebral
edema when cells have insufficient
time to adapt to the hypotonic extra-
cellular environment. In chronic hy-
ponatremia brain cell adaptation has
occurred and, in this setting, an acute
increase in extracellular tonicit y in-
duced by treatment can cause osmotic
demyelination syndrome (ODS).
60,61
Therefore, for each patient with profound
hyponatremi a (S
Na
,125 mmol/L), it is
useful to consider whether cerebral
edema or ODS should be suspected.
62,63
This automatically leads to the often
heated debate on optimal correction
rates in hyponatremia.
64
Both guidelines
reached consensus that the limit (not the
goal) should be around 10 mmol/L per
day for both acute and chronic hypona-
tremia (Table 2).
7,9
Of note, the United
States guideline recommends a lower
limit of 8 mmol/L per day if there is a
high risk of ODS (e.g., in patients with
hypokalemia, alcoholism, malnutrition,
or liver disease).
9
In response to these
recommendations, Adrogué and Madias
proposed even more conservative limits
of 6–8 mmol/L per day regardless of du-
ration or sympto ms.
11
Although we agree
that this is likely to be both sufficient and
safe, the data to support this are still lim-
ited. It is of interest to see how over the
years the recommended correction rates
have gradually become more conserva -
tive (with recommended correction rates
as hig h as 20 mmol/L per day around
1990).
65
A subject directly related to cor-
rection rates is overcorrection. Both
guidelines recommend frequent moni-
toring of S
Na
during the active correction
phase (i.e., all treatments except fluid re-
striction). An aspect that was overlooked
by both guidelines is that the measure-
ment of S
Na
may not offer the precision
required for this monitoring. Tormey
et al. calculated the so-called “reference
change value” for S
Na
using a common
analyzer and demonstrated that only
changes in S
Na
$4 mmol/L were certain
to be real.
12
If overcorrection is detected,
both guidelines used different criteria for
when to relower S
Na
: when initial S
Na
was
,120 mmol/L (United States guideline)
or when limits are exceeded (European
guideline, Table 2). Both guidelines rec-
ommend hypotonic fluids or desmop res-
sin for relowering S
Na
. A combination of
Table 2. Comparison of the United States and European guidelines
Subject United States Guideline European Guideline
Acute or symptomatic
hyponatremia
Severe symptoms: Bolus 3% NaCl
(100 ml over 10 min 3 3 as needed)
Severe symptoms: Bolus 3%
NaCl (150 ml over 20 min 2–3
times as needed)
Moderate symptoms: Continuous
infusion 3% NaCl (0.5–2 ml/kg per h)
Moderate symptoms: Bolus 3%
NaCl (150 ml 3% over 20 min once)
Chronic hyponatremia
SIAD Fluid restriction (first line) Fluid restriction (first line)
Demecl ocycline, urea, or vaptan (second line) Urea or loop diuretics + oral
NaCl (s econd line)
Do not recommend or recommend
against vaptan
a
Recomme nd against lithium
or demeclocycline
Hypovolemic hyponatremia Isotonic saline Isotonic saline or balanced
crystalloid solution
Hypervolemic hyponatremia Fluid restriction Fluid restriction
Vaptans
b
Recomme nd against vapta n
Correction rates Minimum: 4–8 mmol/L per d,
4–6 mmol /L per d (high risk of ODS)
No minimum
Limits: 10–12 mmol/L per d,
8 mmol/L per d (high risk of ODS)
Limit: 10 mmol/L per d
Management of overcorrection Baseline S
Na
$120 mmol/L:
probably unnecessary
Start once limit is exceeded
Baseline S
Na
,120 mmol/L:
start reloweri ng with electrolyte-fre e
water or desmopressin after
correction exceeds 6–8 mmol/L per d
Consult an expert to discuss
infusion containing electrolyte-free
water (10 ml/kg) with or without 2 mg
desmopressin iv
a
“Do not recommend” when S
Na
,130 mmol/L, “recommend against” when S
Na
,125 mmol/L.
b
In li ver cirrhosis, restrict to pa tients where potential benefit outweighs risk of worsened liver fu nction.
9
1344 Journal of the A merican Society of Nephrology J Am Soc Nephrol 28: 1340–1349, 2017
BRIEF REVIEW www.jasn.org