http://www.diva-portal.org
Preprint
This is the submitted version of a paper published in Journal of Physical Chemistry A.
Citation for the original published paper (version of record):
Harper, J K., Tishler, D., Richardson, D., Lokvam, J., Pendrill, R. et al. (2013)
Solid-State NMR Characterization of the Molecular Conformation in Disordered Methyl alpha-
L-Rhamnofuranoside.
Journal of Physical Chemistry A, 117(26): 5534-5541
http://dx.doi.org/10.1021/jp4036666
Access to the published version may require subscription.
N.B. When citing this work, cite the original published paper.
Permanent link to this version:
http://urn.kb.se/resolve?urn=urn:nbn:se:su:diva-92801

1
Solid-state NMR Characterization of Molecular Conformation in Disordered Methyl α–
L–rhamnofuranoside.
James K. Harper,*
a
Derek Tishler,
b
David Richardson,
a
John Lokvam,
c
Robert Pendrill,
d
Göran Widmalm.
d
a
University of Central Florida, Department of Chemistry, 4000 Central Florida Blvd.,
Orlando, FL 32816, USA.
b
University of Central Florida, Department of Physics, Orlando, FL 32816, USA.
c
University of California Berkeley, Department of Biology, Berkeley, CA 94720, USA.
d
Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, S-106
91, Stockholm, Sweden.

2
Solid-state NMR Characterization of Molecular Conformation in Disordered Methyl α–
L–rhamnofuranoside.
Abstract.
A combination of solid-state
13
C NMR tensor data and DFT computational
methods are utilized to predict conformation in disordered methyl α–L–
rhamnofuranoside. This previously uncharacterized solid is found to be crystalline and
consists of at least six distinct conformations that exchange on the kHz timescale. A total
of 66 model structures were evaluated and six were identified as being consistent with
experimental
13
C NMR data. All feasible structures have very similar heavy atom
positions and differ most significantly in OH hydrogen orientations. A concerted
rearrangement of OH hydrogens is proposed to account for the observed dynamic
disorder. This rearrangement is accompanied by smaller changes in ring conformation
and is slow enough to be observed on the NMR timescale due to severe steric crowding
among ring substituents. The relatively minor heavy atom differences in the final
structures suggest that characterization of a complete crystal structure by x-ray powder
diffraction may be feasible.
Keywords.
13
C tensor principal values, NMR crystallography.

3
Introduction.
For the past century the insights provided by crystallography have help guide the
development of science in a remarkably wide range of disciplines. Several well-
established diffraction techniques are now available for determining structure in materials
that form crystals and, to a lesser extent, microcrystalline powders. Recently, the
methods of solid-state NMR have been directed toward the problem of crystallographic
characterization and the prospect of performing “NMR crystallography” has become
feasible.
1
Presently, most NMR crystallographic studies emphasize the NMR
characterization of the molecular structure of an individual molecule or the repeating unit
in framework materials.
2
The longer-range lattice order needed to identify a space group
is usually obtained independently from x-ray powder diffraction methods that rely on the
NMR determined structure as a starting model. However, alternative methods
3
including
theoretical crystal structure prediction methods have also been found to be capable of
also providing the lattice structure.
2b,4
Crystallographic analysis by NMR spectroscopy is appealing because NMR is
capable of characterizing a diverse variety of solids that can be difficult to treat by
conventional diffraction methods. A key early development in the pursuit of crystal
structure by NMR was the ability to characterize molecular conformation by solid-state
NMR.
5
Such structural characterizations can now be achieved using a variety of methods
and have been used to elucidate structure in proteins,
6
inorganic materials
2a,2b,2h,2i,2l,2m
and
smaller organic molecules.
7
Presently, these studies have largely been limited to well-
ordered crystalline solids and extension to more challenging materials is desirable. For
example, many materials form solids containing molecules that are partially disordered or

4
that consist of mixtures of several lattice types (i.e. mixed phase materials). In these
cases NMR has the potential to provide molecular conformation for each unique structure
found in the solid because several resonances are usually observed for each atomic
position due to the multiple distinct conformations present in the solid.
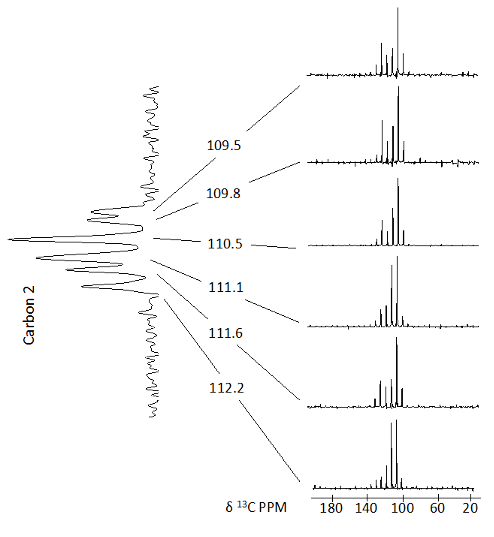
Solid-state NMR is remarkably sensitive to even minor differences in structure
and such variations, when present, often result in new resonances for a given site. Thus,
disordered or mixed phase solids usually exhibit several resonances for each atom in the
molecule. Conformational characterization for each unique structure in these solids is
valuable because such structures provide the initial models needed for crystal structure
determination by powder diffraction methods. Accordingly, the aim of the present study
is to characterize the molecular conformations of one such disordered solid, namely,
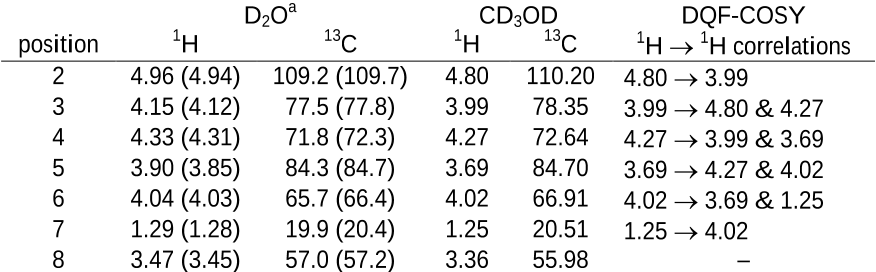
methyl α–L–rhamnofuranoside (Figure 1). Presently, there is no known crystal structure
for methyl α–L–rhamnofuranoside and this study will provide the structure of the
crystallographic asymmetric unit for each conformation present. Inspection of the
13
C
NMR spectrum of solid methyl α–L–rhamnofuranoside shows disorder at several of the
carbons and narrow lines characteristic of a crystalline solid. Here,
13
C tensor principal
values are measured for all sites. Assignment of conformation is accomplished using a
previously described approach
5a
that evaluates a wide variety of possible conformations
and retains structures having computed tensors that agree with experimental data.