
University of Dundee
Neuropathic pain
Finnerup, Nanna B.; Haroutounian, Simon; Kamerman, Peter; Baron, Ralf; Bennett, David L.
H.; Bouhassira, Didier
Published in:
Pain
DOI:
10.1097/j.pain.0000000000000492
Publication date:
2016
Licence:
CC BY
Document Version
Publisher's PDF, also known as Version of record
Link to publication in Discovery Research Portal
Citation for published version (APA):
Finnerup, N. B., Haroutounian, S., Kamerman, P., Baron, R., Bennett, D. L. H., Bouhassira, D., Cruccu, G.,
Freeman, R., Hansson, P., Nurmikko, T., Raja, S. N., Rice, A. S. C., Serra, J., Smith, B. H., Treede, R-D., &
Jensen, T. S. (2016). Neuropathic pain: an updated grading system for research and clinical practice. Pain,
157(8), 1599-1606. https://doi.org/10.1097/j.pain.0000000000000492
General rights
Copyright and moral rights for the publications made accessible in Discovery Research Portal are retained by the authors and/or other
copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with
these rights.
• Users may download and print one copy of any publication from Discovery Research Portal for the purpose of private study or research.
• You may not further distribute the material or use it for any profit-making activity or commercial gain.
• You may freely distribute the URL identifying the publication in the public portal.
Take down policy
If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately
and investigate your claim.
Download date: 25. Aug. 2022

Comprehensive Review
Neuropathic pain: an updated grading system for
research and clinical practice
Nanna B. Finnerup
a,
*, Simon Haroutounian
b
, Peter Kamerman
c
, Ralf Baron
d
, David L.H. Bennett
e
,
Didier Bouhassira
f,g
, Giorgio Cruccu
h
, Roy Freeman
i
, Per Hansson
j,k
, Turo Nurmikko
l
, Srinivasa N. Raja
m
,
Andrew S.C. Rice
n,o
, Jordi Serra
p
, Blair H. Smith
q
, Rolf-Detlef Treede
r
, Troels S. Jensen
a,s
Abstract
The redefinition of neuropathic pain as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system,”
which was suggested by the International Association for the Study of Pain (IASP) Special Interest Group on Neuropathic Pain (NeuPSIG)
in 2008, has been widely accepted. In contrast, the proposed grading system of possible, probable, and definite neuropathic pain from
2008 has been used to a lesser extent. Here, we report a citation analysis of the original NeuPSIG grading paper of 2008, followed by an
analysis of its use by an expert panel and recommendations for an improved grading system. As of February, 2015, 608 eligible articles
in Scopus cited the paper, 414 of which cited the neuropathic pain definition. Of 220 clinical studies citing the paper, 56 had used the
grading system. The percentage using the grading system increased from 5% in 2009 to 30% in 2014. Obstacles to a wider use of the
grading system were identified, including (1) questions about the relative significance of confirmatory tests, (2) the role of screening tools,
and (3) uncertainties about what is considered a neuroanatomically plausible pain distribution. Here, we present a revised grading
system with an adjusted order, better reflecting clinical practice, improvements in the specifications, and a word of caution that even the
“definite” level of neuropathic pain does not always indicate causality. In addition, we add a table illustrating the area of pain and sensory
abnormalities in common neuropathic pain conditions and propose areas for further research.
Keywords: Neuropathic pain, Definition, Grading, Possible, Probable, Definite
1. Introduction
In 1994, the International Association for the Study of Pain (IASP)
defined neuropathic pain as “pain initiated or caused by a primary
lesion or dysfunction in the nervous system.” In 2008, a task force
initiated by the IASP Special Interest Group on Neuropathic Pain
(NeuPSIG) noted the need to distinguish neuropathic pain from
nociceptive pain arising indirectly from neurological disorders and
pain conditions with secondary neuroplastic changes occurring in
the nociceptive system, and proposed a new definition that omitted
the term “dysfunction”: “pain arising as a direct consequence of
a lesion or disease affecting the somatosensory system.”
30
A
slightly modified version of this definition was proposed by the IASP
Taxonomy Committee and accepted by the IASP: “pain caused by
a lesion or disease of the somatosensory nervous system.”
16,17
The omission of the term “dysfunction” excludes conditions
involving ill-defined changes in the nervous system and conditions
with no known lesion of the somatosensory nervous system from
being classified as neuropathic pain. The restriction to the
somatosensory nervous system is important because conditions
such as musculoskeletal pain (eg, due to spasticity) arising
indirectly from disorders of the motor system should not be
confused with neuropathic pain. The term “primary” was omitted
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
a
Danish Pain Research Center, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark,
b
Division of Clinical and Translational Research, Department of
Anesthesiology, Washington University School of Medicine, St. Louis, MO, USA,
c
Brain Function Research Group, School of Physiology, Faculty of Health Sciences, University of
the Witwatersrand, Johannesburg, South Africa,
d
Division of Neurological Pain Research and Therapy, Department of Neurology, Universit ¨atsklinikum Schleswig-Holstein,
Campus Kiel, Kiel, Germany,
e
Nuffield Department of Clinical Neuroscience, University of Oxford, Oxford, United Kingdom,
f
INSERM U-987, Centre d’Evaluation et de Traitement
de la Douleur, CHU Ambroise Par ´e, Boulogne-Billancourt, France,
g
Universit ´e Versailles-Saint-Quentin, Versailles, France,
h
Department of Neurology and Psychiatry, Sapienza
University, Rome, Italy,
i
Autonomic and Peripheral Nerve Laboratory, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA,
USA,
j
Department of Pain Management and Research, Division of Emergencies and Critical Care, Oslo University Hospital, Oslo, Norway,
k
Department of Molecular Medicine and
Surgery, Karolinska Institutet, Stockholm, Sweden,
l
Pain Research Institute, Neuroscience Research Centre, The Walton Centre NHS Foundation Trust, Liverpool, United
Kingdom,
m
Division of Pain Medicine, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA,
n
Pain Research,
Department of Surgery and Cancer, Faculty of Medicine, Imperial College London, United Kingdom,
o
Pain Medicine, Chelsea and Westminster Hospital NHS Foundation Trust,
London, United Kingdom,
p
Neuroscience Technologies, Ltd, Barcelona, Spain,
q
Ninewells Hospital and Medical School, Division of Population Health Sciences, School of
Medicine, University of Dundee, Dundee, Scotland,
r
Chair of Neurophysiology, Center of Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim,
Heidelberg University, Germany,
s
Department of Neurology, Aarhus University Hospital, Aarhus, Denmark
*Corresponding author. Add ress: Danish Pain Research Center, Aarhus University, Aarhus University Hospital, Norrebrogade 44, Building 1A, DK-8000 Aarhus C, Denmark.
Tel.: 145 7846 4230; fax: 145 7846 3269. E-mail address: finnerup@clin.au.dk (N. B. Finnerup).
PAIN 157 (2016) 1599–1606
© 2016 International Association for the Study of Pain. This is an open access article distributed under the terms of the Creative Commons Attribution License 4.0 (CC BY),
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
http://dx.doi.org/10.1097/j.pain.0000000000000492
August 2016
·
Volume 157
·
Number 8 www.painjournalonline.com 1599
because of the difficulty in distinguishing between primary and
secondary causes of neuropathic pain; however, the omission
means that nociceptive pain conditions that—over time—may
cause secondary lesions in the somatosensory nervous system
could ultimately be considered as being partly neuropathic pain.
Recognizing the challenges of determining the presence of
neuropathic pain according to this new definition, NeuPSIG also
proposed a grading system
30
to guide decisions on the level of
certainty with which neuropathic pain can be determined in an
individual patient. Three levels of certainty—possible, probable,
and definite neuropathic pain—were proposed. As an activity in the
Global Year Against Neuropathic Pain,
15
NeuPSIG established
a committee to (1) critically evaluate the use of the grading system in
the 7 years after its publication, (2) assess the usefulness and
limitations of the grading system, and (3) update the grading system
if required, for improved application in clinical and research settings.
The committee consisted of an expert panel of neurologists, clinical
neurophysiologists, neuroscientists, anesthesiologists, pain spe-
cialists, primary care physicians, and population health scientists.
2. Procedure
To generate background material and discussion points, we
performed a systematic literature search using the Scopus
database, which is an abstract and citation database of peer-
reviewed literature (including scientific journals, books, and confer-
ence proceedings). This database was searched on February 6,
2015 for publications that cited the original NeuPSIG grading paper
from 2008.
30
Three review authors (S.H., P.K., and N.B.F.) extracted
the following data: (1) use of the citation, (2) classification of the
publication as a review, animal or human experimental paper,
a clinical study, or others, (3) criteria used for including or classifying
patients with neuropathic pain in c linical studies, (4) comparison of
the grading with other criteria for identifying neuropathic pain when
available, and (5) any issues raised with the grading system.
In addition, all committee members were asked to examine the
grading system for possible deficiencies that could require
modification or amendment in a subsequent iteration of the grading
system. Participants convened under the auspices of NeuPSIG in
Nice, France, on May 14, 2015. Before the meeting, all participants
were provided with a documentation folder that included results from
the literature review and issues identified by committee members. At
the meeting, data on the use of the grading paper were presented,
and individual participants provided short overviews on issues with
the grading system that had been identified before the meeting.
Discussions pertaining to the issues identified before the meeting
and new issues raised by participants at the meeting were used to
inform modifications to the existing grading system. Before and after
the meeting, the process and update were discussed through
e-mail, and after circulating draft manuscripts through e-mail, a final
update was agreed through consensus by e-mail.
3. Background material
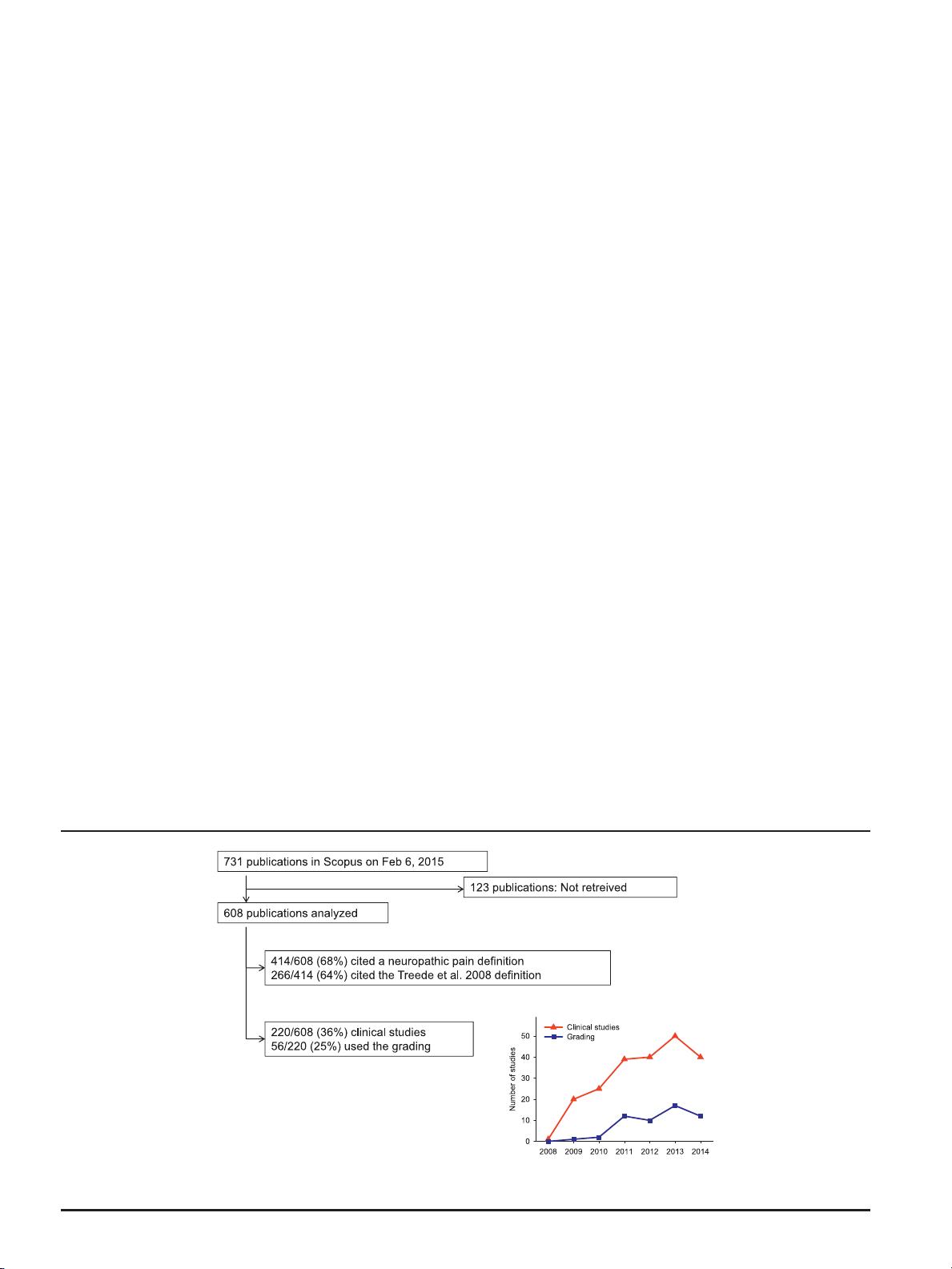
A total of 731 publications were identified in Scopus as citing the
original grading paper
30
at the time of search, which represented
about 5% of all publications in Scopus with the term “neuropathic
pain” in the title, abstract, or keywords in the same period. Of the
731 publications, 123 were not available as full-text at any of the 3
institutions, at which the reviewers were based or were in
a language not understood by the reviewers. Hence, the full text
of 608 publications was downloaded and used to evaluate the
use of the original grading paper since its publication in 2008. Of
the 608 included publications, 269 were classified by the
reviewers as reviews or book chapters, 220 as clinical studies,
73 as experimental studies, and 46 as “others.”
Of the 608 publications, 414 cited the grading paper
30
in
relation to the definition of neuropathic pain (Fig. 1). Of these, 266
used the definition as it was presented (or very similar) in the
original grading paper, whereas 48 applied the adapted 2011
IASP definition
17
and 8 applied the 1991 IASP definition despite
using the grading paper as the reference. Ninety-two presented
other definitions of neuropathic pain, of which most had a wording
consistent with the definition in the grading paper, whereas others
presented a definition significantly different despite using the
grading paper as reference. The grading paper was cited in
relation to other statements, unrelated to the definition or grading
system, in 190 publications.
Of the 220 clinical publications that included patients, only 56
(25% of clinical studies, 9% of all studies citing the grading paper)
used the grading system to include or classify patients as having
possible, probable, or definite neuropathic pain. A further 16 (7%)
Figure 1. Summary of how the citations of the neuropathic pain grading paper
30
were used. The figure indicates the percentage of 608 publications that cited the
original grading paper
30
for defining neuropathic pain and the number of clinical studies that used the grading system for identifying neuropathic pain. The insert
indicates the total number of clinical studies and the number of studies using the grading system
30
for identifying neuropathic pain per year.
1600 N.B. Finnerup et al.
·
157 (2016) 1599–1606 PAIN
®

used other criteria for classification of pain, but retrospectively
noted whether the patients had possible, probable, or definite
neuropathic pain according to the grading system. The percent-
age of clinical studies citing the grading system that also used it to
include or classify patients with neuropathic pain increased from
5% (1/20) in 2009 to 30% (12/40) in 2014 (Fig. 1). Of the
remaining 148 studies that did not use the grading system for
patient classification, 115 used other criteria to include or classify
neuropathic pain patients. Of these, 50 used one or more
questionnaires, 30 used Douleur Neuropathique en 4 questions,
11 used painDETECT, 8 LANSS (Leeds Assessment of Neuro-
pathic Symptoms and Signs) or S-LANSS (self-report LANSS),
2 used McGill pain questionnaire, 1 used ID-Pain, 1 used
standardized evaluation of pain, and 1 used the German pain
questionnaire; 51 used various criteria including pain history, pain
descriptors, clinical examination, and laboratory investigations; 2
used patient self-report; and 12 did not mention the criteria used.
Thus, the 2008 grading paper was mostly cited for the redefinition
of neuropathic pain. The redefinition has since been introduced in
the IASP terminology with minor modifications, and hence the
authors’ aim “to develop a more precise definition of neuropathic
pain that will be useful for clinical and research purposes” has largely
been achieved. The adoption of the grading system has naturally
happened after a delay, and since 2011 a steady ratio of about 1/3 of
clinical trials in the field have used it (Fig. 1).
A meta-analysis of cancer trials
4
indicated that of the 4 criteria of
the grading system, criterion 2 “a history suggestive of a relevant
lesion or disease affecting the peripheral or central somatosensory
system” and criterion 3 “demonstration of the distinct neuro-
anatomically plausible distribution by at least 1 confirmatory test”
were available in the majority of trials (13-14 of 22), whereas
criterion 1 “pain with a distinct neuroanatomically plausible
distribution” was available less often (10/22) and confirmation of
the underlying lesion of disease was rarely done (criterion 4, 7/22).
This identifies 2 problems to be addressed in the present revision:
(1) plausibility of pain distribution and its assessment and (2) need
for establishing the neurological diagnosis by confirmatory tests.
In addition, the following deficiencies were identified from the
paper reviews and discussed during the committee meeting: (1)
Several screening tools (questionnaires) were developed before the
redefinition of neuropathic pain by NeuPSIG and IASP
3,13
but are
not positioned in the grading system; (2) Some clinicians and
investigators have difficulty in determining the topographical
location of a lesion and its pathology, as the approach used in
neurology of “where is the lesion?”, “what is the lesion?” is not
intuitive to other medical disciplines; (3) Certain sensory signs are
not specific to neuropathic pain; and (4) Determination of lesion
type and location does not necessarily prove that the pain is caused
by that lesion or disease (uncertainty of causal relationship).
Based on these limitations of the current grading system, we
propose a change to the order of the grading criteria to better
reflect clinical practice and have furthermore annotated the terms
used to improve clarity (Fig. 2). In addition, a research agenda is
proposed to further address shortcomings of the grading system.
Figure 2. Flow chart of updated grading system for neuropathic pain.
a
History, including pain descriptors, the presence of nonpainful sensory symptoms, and
aggravating and alleviating factors, suggestive of pain being related to a neurological lesion and not other causes such as inflammation or non-neural tissue
damage. The suspected lesion or disease is reported to be associated with neuropathic pain, including a temporal and spatial relationship representative of
the condition; includes paroxysmal pain in trigeminal neuralgia.
b
The pain distribution reported by the patient is consistent with the suspected lesion or disease
(Table 1).
c
The area of sensory changes may extend beyond, be within, or overlap with the area of pain. Sensory loss is generally required but touch-evoked or
thermal allodynia may be the only finding at bedside examination. Trigger phenomena in trigeminal neuralgia may be counted as sensory signs. In some cases,
sensory signs may be difficult to demonstrate although the nature of the lesion or disease is confirmed; for these cases the level “probable” continuestobe
appropriate, if a diagnostic test confirms the lesion or disease of the somatosensory nervous system.
d
The term “definite” in this context means “probable
neuropathic pain with confirmatory tests” because the location and nature of the lesion or disease have been confirmed to be able to explain the pain. “Definite”
neuropathic pain is a pain that is fully compatible with neuropathic pain, but it does not necessarily establish causality.
August 2016
·
Volume 157
·
Number 8 www.painjournalonline.com 1601

4. Revised grading system
The grading system is intended for determining the level of
certainty with which the pain in question is neuropathic. A finding
of probable neuropathic pain in a given individual patient should
prompt consideration of treatment according to the neuropathic
pain treatment guidelines,
10
but the grading system is not
intended for medico-legal purposes or to classify diseases. The
refinements in the present grading system (Fig. 2) follow the
classical clinical method of diagnosis in that history, clinical
examination, and diagnostic tests stepwise add to level of
certainty that the pain in question is neuropathic.
4.1. Possible neuropathic pain
Evaluation of the patient according to the grading system should be
undertaken if the patient’s history suggests that pain could be
related to a neurological lesion or disease and not other causes
such as inflammation or non-neural tissue damage. At this stage,
pain descriptors, the presence of nonpainful sensory symptoms,
and any aggravating and alleviating factors can be taken into
account. Pain descriptions such as burning or hot, electric shocks
or shooting, pricking or pins and needles, pain evoked by light
touching or cold, and nonpainful sensations such as numbness and
tingling are suggestive, but not pathognomonic for neuropathic
pain, and other descriptors may apply as well.
3
The combination of
several descriptors, however, has a highly discriminant value and
several screening tools (questionnaires) have been developed to
identify patients who may have neuropathic pain to alert the clinician
to undertake further assessment (though they cannot be used
alone to identify neuropathic pain).
3,13,32
Theseinclude,butarenot
limited to the LANSS,
2
the neuropathic pain questionnaire,
18
the
Douleur Neuropathique en 4 questions,
6
the painDETECT,
11
and
ID-Pain.
24
The following two criteria need to be fulfilled to reach the first
level of certainty-“possible” neuropathic pain.
4.1.1. A history of relevant neurological lesion or disease
There should be a clinical suspicion of a relevant lesion or disease
of the somatosensory nervous system (eg, an episode of acute
herpes zoster or a traumatic nerve injury). The temporal relationship
between the lesion or disease and the pain may vary, but a close
temporal relationship helps strengthen the clinical suspicion. The
onset of pain is usually immediate or within a few weeks of the
lesion or disease but may be delayed for up to several months after
injury (eg, after stroke) or for many years in conditions with an
insidious onset such as diabetic neuropathy. In some cases, the
history of pain or sensory disturbances by themselves suggest
a disease, eg, in polyneuropathy (peripheral neuropathy), where the
insidious onset of distal pain or numbness may be the only history
indicating the disease. Characteristic sudden short-lasting (usually
a few seconds) paroxysmal pain in the face, which may recur
several times and may be separated by a refractory period (usually
some minutes), suggests trigeminal neuralgia, where the pain is the
only symptom indicating a relevant neurological disease.
4.1.2. Pain distribution neuroanatomically plausible
The pain distribution should be anatomically consistent with the
suspected location of the lesion or disease in the peripheral or
central somatosensory nervous system (as derived from the
patient’s history). This can be difficult to decipher in the single
patient, as the distribution of pain can occupy a smaller area or
extend somewhat outside the innervation territory of a peripheral
nerve or root or the somatotopic representation of the body within
the central nervous system, but it should be in a distribution that is
typical for the underlying disorder (see examples in Table 1). In
painful channelopathies, the pain distribution may be unusual but
should be consistent with the disorder, eg, familial episodic pain
syndrome, in which pain is mainly localized to the chest and upper
arms, or inherited erythromelalgia, in which pain is localized to the
extremities (feet and hands and in some cases ears).
When both requirements 1 and 2 of the pain history are fulfilled,
the pain complaint may be termed possible neuropathic pain.
4.2. Probable neuropathic pain
The next level of certainty requires supporting evidence obtained
by a clinical examination. The examination should optimally
confirm the presence of negative sensory signs, ie, partial or
complete loss to one or several sensory modalities concordant
with the lesion or disease of the somatosensory nervous system
(eg, light touch, cold temperature, Tables 1 and 2).
Demonstrating sensory loss to one or more of these modalities
and delineation of the area affected by the negative sensory
phenomena are central to the determination as to whether
a nervous system lesion is the cause of the sensory disturbance
(ie, whether it is compatible with neuropathy). Negative sensory
signs may also be seen in nociceptive pain, but in these cases
they lack neuroanatomically distinct borders and are not re-
producible.
12,19
The sensory signs may or may not be accom-
panied by motor or autonomic signs.
Positive sensory signs alone (eg, pressure-evoked hyperalgesia)
carry less weight towards neuropathic pain probability, in
particular, if their distribution does not follow relevant neuroana-
tomical delineation. Positive sensory symptoms and signs may be
seen in patients with other conditions such as inflammatory pain,
pain of unknown origin, anxiety, and sleep deprivation, and can be
affected by stress and negative emotions.
25,34
It is important to
emphasize that there are conditions where sensory loss is not
a prerequisite for a neuropathic pain condition. In certain
neuropathic pain conditions such as hereditary channelopa-
thies
1,33
and in subgroups of patients with, eg, peripheral nerve
injury,
20
touch-evoked allodynia or thermal hyperalgesia may be
present without detectable sensory loss. The presence of such
positive signs may mask sensory loss in some of these patients.
Idiopathic or classical trigeminal neuralgia is a special case. In
trigeminal neuralgia, sensory deficits may not be found on clinical
examination, although quantitative sensory testing may show
sensory abnormality.
20
In these cases, a history of characteristic
triggering maneuvers may be counted as positive sensory signs.
They can sometimes be repeated by the examiner, who may thus
evoke and see the characteristic tic. Another special case is
painful channelopathies as they are often paroxysmal and
sensory examination can be normal between attacks. A history
of characteristic symptoms may be considered a surrogate for
positive sensory signs. In phantom pain, a sensory examination is
not possible in the pain area. In these cases, the loss of the body
part where pain is perceived is counted as a surrogate for sensory
signs within the pain distribution.
Often, sensory changes to light touch, vibration, pinprick, cold,
or warmth can be confirmed by a clinical examination (Table 2),
but more detailed analysis using quantitative sensory testing may
be needed.
13
Prolonged pain after herpes zoster is associated
with sensory abnormalities in a neuroanatomically plausible
distribution in most, but not all cases.
20
In rare cases where
sensory abnormalities are doubtful or lacking, documentation of
a herpes zoster rash in the form of a photograph or clinical record
1602 N.B. Finnerup et al.
·
157 (2016) 1599–1606 PAIN
®