Journal of Radioanalytical and Nuclear Chemistry
1
A comparative study using liquid scintillation counting 1
to determine
63
Ni in low and intermediate level 2
radioactive waste 3
Names of the authors: Céline Gautier
1
, Christèle Colin
1
, Cécile Garcia
1♯
4
Title: A comparative study using liquid scintillation counting to determine
63
Ni in low 5
and intermediate level radioactive waste 6
Affiliation(s) and address(es) of the author(s):
1
Operator Support Analyses Laboratory, 7
Atomic Energy Commission, CEA Saclay, DEN/DANS/DPC/SEARS/LASE, Building 8
459, PC 171, 91191 Gif-sur-Yvette Cedex, FRANCE 9
♯
on leave for
AREVA, Demantelement et Services/MSIS Assistance, 91196 Gif-sur-10
Yvette Cedex, FRANCE
11
E-mail address of the corresponding author: celine.gautier@cea.fr 12
13

Journal of Radioanalytical and Nuclear Chemistry
2
A comparative study using liquid scintillation counting 14
to determine
63
Ni in low and intermediate level 15
radioactive waste 16
Céline Gautier
1
, Christèle Colin
1
, Cécile Garcia
1♯
17
1
Operator Support Analyses Laboratory, Atomic Energy Commission, CEA Saclay, 18
DEN/DANS/DPC/SEARS/LASE, Building 459, PC 171, 91191 Gif-sur-Yvette Cedex, 19
FRANCE 20
♯
On
leave for AREVA, Demantelement et Services/MSIS Assistance, 91196 Gif-sur-Yvette 21
Cedex, FRANCE 22
Abstract 23
A comparative study using liquid scintillation counting was performed to measure
63
Ni in 24
low and intermediate level radioactive waste. Three dimethylglyoxime (DMG)-based 25
radiochemical procedures (solvent extraction, precipitation, extraction chromatography) 26
were investigated, the solvent extraction method being considered as the reference 27
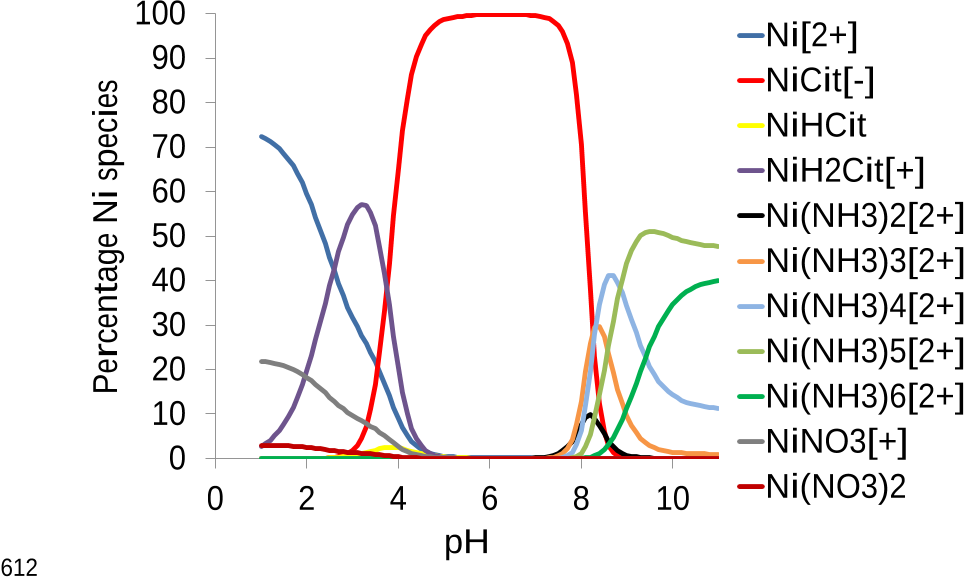
method. Theoretical speciation calculations enabled to better understand the chemical 28
reactions involved in the three protocols and to optimize them. In comparison to the 29
method based on DMG precipitation, the method based on extraction chromatography 30
allowed to achieve the best results in one single step in term of recovery yield and 31
accuracy for various samples. 32
Keywords 33
63
Ni, radiochemical analysis, liquid scintillation counting, decommissioning, radioactive 34
waste, dimethylglyoxime 35

Journal of Radioanalytical and Nuclear Chemistry
3
Introduction 36
In France, the National Radioactive Waste Management Agency (ANDRA) is in 37
charge of the long-term management of all radioactive waste. Several repository sites 38
have been built in order to accommodate nuclear waste packages. One is dedicated to the 39
Low and Intermediate Level short-lived Waste. The specifications for 143 radionuclides 40
have been defined by ANDRA which guarantees the safety of the facility [1]. Among this 41
long list,
63
Ni has to be declared as soon as its activity concentration is over 1 Bq g
-1
and 42
its maximum acceptance limit has been fixed to 3 x 10
6
Bq g
-1
[1].
63
Ni is produced by 43
neutron activation reactions of stable Ni and Cu which are components of various 44
materials used in the nuclear fuel cycle [2]. Consequently,
63
Ni can be present in many 45
radioactive materials and waste samples [2-17], such as graphites [6, 7], metals 46
(aluminium, lead, steel) [6-11], concretes [6, 7, 10, 12], ion-exchange resins and 47
charcoals [13], effluents [8, 14-17], sludges [14] and environmental samples [10, 18]. 48
63
Ni is a long-lived radionuclide with a half-life of 98.70 years (±24) [19]. It is a pure 49
beta emitter with a maximum energy of 66.98 keV [19]. As liquid scintillation counting 50
(LSC) has a high counting efficiency for
63
Ni (around 70 %) [2], this detection technique 51
is widely used for
63
Ni determination [2-17]. As a pure beta emitting radionuclide,
63
Ni 52
must be isolated from the matrix and the interfering radionuclides (especially
60
Co a 53
major radionuclide which has a similar chemical behavior) through chemical separations 54
prior to any analysis by LSC [2-17]. Consequently, a selective radiochemical method is 55
needed to measure
63
Ni in low and intermediate level radioactive waste [2-18]. Most 56
procedures of
63
Ni purification rely on the complexing agent of dimethylglyoxime 57
(DMG) implemented in three different types of methods: solvent extraction, precipitation 58
and extraction chromatography [2-18]. In all cases, the Ni(DMG)
2
complex is favourably 59
formed at basic pH, around 8-9 [2-18]. The recovery yield of the overall radiochemical 60
procedure is generally determined from the measurement of stable Ni by atomic 61
absorption spectroscopy (AAS) [12] or inductively coupled plasma - atomic emission 62
spectroscopy (ICP-AES) [5, 13, 15, 17]. 63

Journal of Radioanalytical and Nuclear Chemistry
4
Two or three decades ago, the reference radiochemical method to analyse
63
Ni was 64
based on a liquid-liquid extraction procedure. The Ni(DMG)
2
complex is first extracted 65
in an organic solvent [20], commonly chloroform [8, 10, 11, 18, 20] which has a higher 66
Ni extraction capacity [20]. Ni is then back-extracted in aqueous solution, mostly with 67
hydrochloric acid [11, 16, 18]. In France, this extraction method has been standardized in 68
the standard NF M60-317 to determine
63
Ni in radioactive effluents and waste [21]. Ni 69
amount is generally less than 1 mg [8, 18, 20] whereas the DMG amount varies from 10 70
mg [20] to 250 mg [8]. By replicating several extractions, this type of separation 71
procedure enabled to achieve satisfactory decontamination factors of Co towards Ni (less 72
than 0.2% of Co was extracted) [8]. In spite of its efficiency, the implementation of this 73
solvent extraction procedure has tended to decrease in the last decades because of the 74
restrictions of chloroform use, notably through the European REACH regulation [22]. 75
An alternative method to solvent extraction is the precipitation of the Ni(DMG)
2
76
complex [4, 9, 12-14]. The French standard NF M60-317 also includes this alternative 77
option as a second
63
Ni purification method [21]. When the total activity concentrations 78
of the other radionuclides are 10 times higher in comparison to
63
Ni, this standard 79
indicates the necessity to perform a second precipitation step [21]. Higher Ni amount is 80
added (around 2 or 3 mg) [12-14] whereas the DMG amount varies from 50 mg [12, 13] 81
to 200 mg [21] to favour the precipitation of the Ni(DMG)
2
complex, in comparison to 82
the solvent extraction method. Prior to LSC, the precipitate is destroyed to recover
63
Ni in 83
solution by using concentrated nitric acid [4, 9, 12, 13] or hydrogen peroxide [14]. The 84
procedure based on Ni(DMG)
2
precipitation has been applied for the measurement of 85
63
Ni in various radioactive matrices [4], such as metals [9], concretes [12], ion exchange 86
resins [13] and sludges [14]. However, the destruction of Ni(DMG)
2
precipitate appears 87
to be a delicate and fastidious step before LSC analysis [21]. 88
To overcome these above problems, the technique of extraction chromatography 89
based on the Eichrom Ni
®
resin has been developed to isolate Ni from the interfering 90
elements [23]. Some authors also prepared in-house Ni resins which relies on the same 91
principle [15, 27]. Indeed, over the past 20 decades, extraction chromatography has 92
become a leading technique for separation and preconcentration of radionuclides in the 93

Journal of Radioanalytical and Nuclear Chemistry
5
environmental, biological and nuclear fields [24, 25]. The combination of an organic 94
extractant coated on an inert support delivers the selectivity of solvent extraction with the 95
ease of use of resin based methods. In the case of Ni resin, the DMG extractant is coated 96
on an inert support of acrylic ester based-resin [23]. As relatively high amounts of DMG 97
and Ni are involved (respectively 50 mg and 2 to 3 mg for a 2 mL pre-packed column 98
[23]), on-column precipitation of Ni with DMG occurs on Ni resin [23]. Elimination of 99
the interfering elements is mainly achieved with ammonium citrate during the rinsing 100
step. Then, Ni is generally stripped from the column using nitric acid [23, 26]. In recent 101
years, many radiochemical procedures based on Ni resin have been applied on many 102
nuclear materials [5, 6, 12, 13, 15, 17, 27]. 103
DMG is an effective and selective complexing agent of Ni but also of other metal 104
elements, such as Co, Cu, Cd and Pd [28], which can induce interferences for
63
Ni 105
purification. Indeed, the
60
Co activation product is often present in substantial amounts in 106
radioactive materials in comparison to
63
Ni. Correlation factors between
63
Ni and
60
Co 107
highly depend on the types of nuclear plants and samples [29]. In CEA France, the third 108
quartile of
63
Ni/
60
Co ratio has been determined at 0.4 in solid radioactive waste. 109
Consequently, from the literature, it is frequently necessary to complete the purification 110
step based on DMG with other separation procedures so as to eliminate Co efficiently. In 111
the French standard NF M60-317, the elimination of Co is achieved with a preliminary 112
liquid-liquid extraction step based on the use of 2-nitroso-1-naphthol [21]. In this 113
standard, it is recommended to implement this Co solvent extraction when the total 114
activity concentrations of the other radionuclides are 10 times higher in comparison to 115
63
Ni [21]. Furthermore, the presence of
55
Fe, another significant activation product, can 116
also hinder the formation of Ni(DMG)
2
complex/precipitate because of its precipitation at 117
basic pH [23, 26]. Organic complexing agents, such as citric acid [6, 12, 21], tartaric acid 118
[9, 21] or oxalic acid [5] are generally introduced to prevent the precipitation of Fe and 119
the other metal elements at basic pH. However, their chelating properties may not be 120
sufficient in case of high Fe amounts, such as in steels [6, 28]. Consequently, it is also 121
highly recommended to remove Fe to achieve accurate
63
Ni measurements. Precipitation 122
with ammonia [12-16, 18] or hydroxide [6, 14] and anion exchange chromatography [4, 123
5, 9, 10, 11, 14, 15, 17] have been mainly applied in order to eliminate the interfering 124