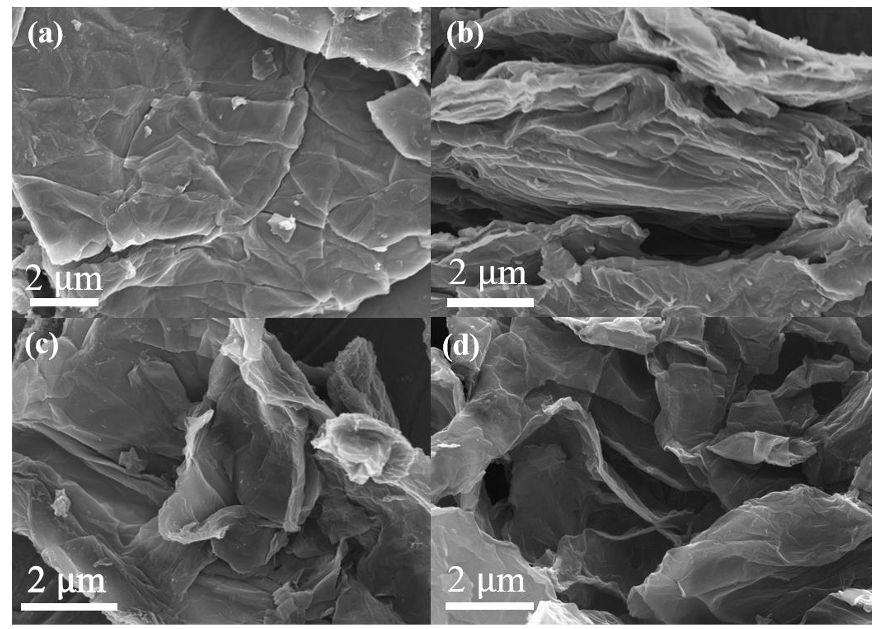
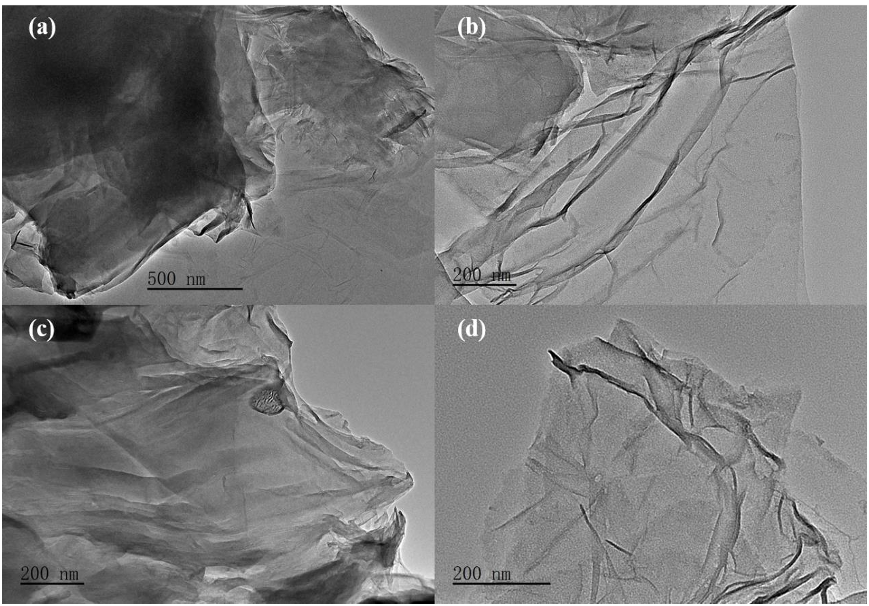
Q2. What is the effect of the annealing process on the graphene?
The annealing process with a slowheating rate (5 °C/min) also effectively inhibited the polycondensation of urea as the process only occurs during the rapidly thermal polymerization in the presence of large amounts of urea.
Q3. What is the effect of acidic conditions on the catalytic performance of metal catalysts?
the acidic/basic condition of the reaction solutions can impact the catalytic performance of metal catalysts as the strong acidic condition can destroy the metal crystalline structure and lead to severe metal leaching.
Q4. What is the common method of introducing heteroatoms into the carbon lattice?
Introducing heteroatoms (B, N, O, P, S, and so forth) into the carbon lattice can effectively disorientate the homogeneously conjugated electron network and modulate the surface properties by tweaking the charge distribution and spinning culture of the doped domains [13, 14].
Q5. What is the effect of N-doping on the carbon network?
Their pioneering studies integrating with material design and theoretical calculations revealed that, due to a greater electronegativity (χN = 3.04) compared with the carbon atom (χC = 2.55), N-doping can effectivtly interrupt the highly-conjugated carbon network of graphene and induce the electron transport from the neighbouring carbon to nitrogen atoms, giving rise to positively charged carbon atoms [37, 38].
Q6. What is the role of N-doping in the synthesis of graphene?
Many studies have reported that N-doping can effectively break the inertness of sp2 hybridized carbon lattice and dramatically tailor the electron density and spin culture of the adjacent carbon, giving rise to the superb catalytic performances in electrochemistry, hydrocarbon conversion, and superoxide activation [34-36].
Q7. What is the effect of the temperature on the annealing of graphene?
Their previous study also indicated raising the annealing temperature in inert atmosphere would result in a lower N-doping level due to the breakup of C-N bond while affording a higher proportion of quaternary N benefiting from the better thermal stability of graphitic N, which is simultaneously bonded with three carbon atoms with substitutional doping in the carbon framework [26].
Q8. What is the effect of the annealing and doping processes on carbon recovery?
The mild annealing and doping processes favored the oxidative atmosphere due to the induced structure defects such as edging sites and vacancies which enabled carbon reconstruction for N-doping.
Q9. What is the rGO's role in preparing graphene?
the rGO can be used as a better carbon precursor to prepare nitrogen-doped graphene with a desirable SSA, N-doping level, defective degree, and stunning catalytic activity.
Q10. What is the effect of annealing graphene?
With respect to rGO, the re-constructing of graphene boundaries (edging sites) and lattice during annealing may also help attain certain amounts of N-doping as shown in this study.
Q11. What is the role of carbocatalysts in the removal of toxic pollutants?
The green and efficient carbocatalysts have demonstrated extraordinary potentials for activating various superoxides (e.g. peroxymonosulfate, persulfate, hydrogen peroxide, and ozone) for the oxidative removal of toxic pollutants in wastewater without any secondary contamination [18-21].
Q12. What is the precursor for nitrogen doping?
Both organic substances and inorganic salts were applied as the nitrogen precursors for nitrogen doping and urea was discovered to be the best precursor with a high doping level and better reducibility without any polycondensation.
Q13. What is the effect of N-doping on the catalytic performance of graphen?
The experimental results indicated that the nitrogen-doped graphene with a higher proportion of graphitic N at a similar doping level exhibited a better catalytic activity for phenol oxidation, and the densities functional theory (DFT) calculations further evidenced that the adsorption of PMS molecules at the adjacent carbon of graphitic N exhibited the lowest adsorption energy and greatest tendency for electron transfer from carbon lattice to PMS for the activation of superoxide O-O bond [26].
Q14. Why was the proportion of graphitic N in NG-Urea-air low?
The proportion of graphitic N of NG-Urea-air and N-rGO-air in total nitrogen was low due to the fact that the mild annealing temperature cannot produce high contents of substitutional N-doping into the carbon lattice.
Q15. What is the way to activate PMS on graphene?
The outstanding efficiency of PMS activation on nitrogen-doped graphene is also contributed by the non-radical process, in which the PMS molecules are activated at N-doped domains and followed by readily oxidation of target organics via electron transfer as illustrated in Fig.
Q16. Why is the mechanism of carbocatalysis unclear?
due to the complicated structure and surface chemistry of nanocarbons, the mechanism of carbocatalysis in metal-free oxidation remains ambiguous, leaving more blanks for mechanisticstudy.
Q17. What is the effect of the increased nitrogen in graphene?
Fig. S8 manifested that the phenol oxidation rate was speeded up with the increased nitrogen amount in graphene, suggesting that the high doping level leads to a promoted catalytic performance for PMS activation.
Q18. What is the effect of the thermal annealing on graphene?
The ammonium salts would decompose during the thermal annealing and release NH3 (the doping agent) and other gasses (N2, N2O, or HCl), which facilitate the formation of porous N-doped graphene with larger SSAs, pore size, and pore volume (Fig. S9 and Table S2).
Q19. What is the effect of thermal annealing on graphene oxide?
The XPS surveys in Fig. 3a illustrate that thermal annealing effectively eliminated the oxygen groups of the graphene oxide (31.4 at.%, Table 1) to form a more reductive surface of rGO-air (14.3 at.%).