
RES E A R C H Open Access
MOG-IgG in NMO and related disorders: a
multicenter study of 50 patients. Part 2:
Epidemiology, clinical presentation,
radiological and laboratory features,
treatment responses, and long-term
outcome
Sven Jarius
1*
, Klemens Ruprecht
2
, Ingo Kleiter
3
, Nadja Borisow
4,5
, Nasrin Asgari
6
, Kalliopi Pitarokoili
3
,
Florence Pache
4,5
,OliverStich
7
, Lena-Alexandra Beume
7
, Martin W. Hümmert
8
, Marius Ringelstein
9
, Corinna Trebst
8
,
Alexander Winkelmann
10
, Alexander Schwarz
1
, Mathias Buttmann
11
, Hanna Zimmermann
2
, Joseph Kuchling
2
,
Diego Franciotta
12
, Marco Capobianco
13
, Eberhard Siebert
14
, Carsten Lukas
15
, Mirjam Korporal-Kuhnke
1
,
Jürgen Haas
1
, Kai Fechner
16
, Alexander U. Brandt
2
, Kathrin Schanda
17
, Orhan Aktas
8
, Friedemann Paul
4,5†
,
Markus Reindl
17†
, and Brigitte Wildemann
1†
; in cooperation with the Neuromyelitis Optica Study Group (NEMOS)
Abstract
Background: A subset of patients with neuromyelitis optica spectrum disorders (NMOSD) has been shown to be
seropositive for myelin oligodendrocyte glycoprotein antibodies (MOG-IgG).
Objective: To describe the epidemiological, clinical, radiological, cerebrospinal fluid (CSF), and electrophysiological
features of a large cohort of MOG-IgG-positive patients with optic neuritis (ON) and/or myelitis (n = 50) as well as attack
and long-term treatment outcomes.
Methods: Retrospective multicenter study.
(Continued on next page)
* Correspondence: sven.jarius@med.uni-heidelberg.de
†
Equal contributors
Brigitte Wildemann, Markus Reindl, and Friedemann Paul are equally
contributing senior authors.
1
Molecular Neuroimmunology Group, Department of Neurology, University
of Heidelberg, Heidelberg, Germany
Full list of author information is available at the end of the article
© The Author(s). 2016 Open Access This article is distributed under the terms of the Creative Commons Attribut ion 4.0
International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and
reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to
the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver
(http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Jarius et al. Journal of Neuroinflammation (2016) 13:280
DOI 10.1186/s12974-016-0718-0

(Continued from previous page)
Results: The sex ratio was 1:2.8 (m:f). Median age at onset was 31 years (range 6-70). The disease followed a multiphasic
course in 80% (median time-to-first-relapse5months;annualizedrelapserate0.92)andresultedinsignificantdisabilityin
40% (mean follow-up 75 ± 46.5 months), with severe visual impairment or functional blindness (36%) and markedly
impaired ambulation due to paresis or ataxia (25%) as the most common long-term sequelae. Functional blindness in
one or both eyes was noted during at least one ON attack in around 70%. Perioptic enhancement was present in several
patients. Besides acute tetra-/paraparesis, dysesthesia and pain were common in acute myelitis (70%). Longitudinally
extensive spinal cord lesions were frequent, but short lesions occurred at least once in 44%. Fourty-one percent had a
history of simultaneous ON and myelitis. Clinical or radiological involvement of the brain, brainstem, or cerebellum was
present in 50%; extra-opticospinal symptoms included intractable nausea and vomiting and respiratory insufficiency (fatal
in one). CSF pleocytosis (partly neutrophilic) was present in 70%, oligoclonal bands in only 13%, and blood-CSF-barrier
dysfunction in 32%. Intravenous methylprednisolone (IVMP) and long-term immunosuppression were often effective;
however, treatment failure leading to rapid accumulation of disability was noted in many patients as well as flare-ups
after steroid withdrawal. Full recovery was achieved by plasma exchange in some cases, including after IVMP failure.
Breakthrough attacks under azathioprine were linked to the drug-specific latency period and a lack of cotreatment with
oral steroids. Methotrexate was effective in 5/6 patients. Interferon-beta was associated with ongoing or increasing
disease activity. Rituximab and ofatumumab were effective in some patients. However, treatment with rituximab was
followed by early relapses in several cases; end-of-dose relapses occurred 9-12 months after the first infusion. Coexisting
autoimmunity was rare (9%). Wingerchuk’s 2006 and 2015 criteria for NMO(SD) and Barkhof and McDonald criteria for
multiple sclerosis (MS) were met by 28%, 32%, 15%, 33%, respectively; MS had been suspected in 36%. Disease onset
or relapses were preceded by infection, vaccination, or pregnancy/delivery in several cases.
Conclusion: Our findings from a predominantly Caucasian cohort strongly argue against the concept of MOG-IgG
denoting a mild and usually monophasic variant of NMOSD. The predominantly relapsing and often severe disease
course and the short median time to second attack support the use of prophylactic long-term treatments in patients with
MOG-IgG-positive ON and/or myelitis.
Keywords: Myelin oligodendrocyte glycoprotein antibodies (MOG-IgG), Autoantibodies, Neuromyelitis optica spectrum
disorders (NMOSD), Aquaporin-4 antibodies (AQP4-IgG, NMO-IgG), Optic neuritis, Transverse myelitis, Longitudinally
extensive transverse myelitis, Magnetic resonance imaging, Cerebrospinal fluid, Oligoclonal bands, Electrophysiology,
Evoked potentials, Treatment, Therapy, Methotrexate, Azathioprine, Rituximab, Ofatumumab, Interferon beta, Glatiramer
acetate, Natalizumab, Outcome, Pregnancy, Infections, Vaccination, Multiple sclerosis, Barkhof criteria, McDonald criteria,
Wingerchuk criteria 2006 and 2015, IPND criteria, International consensus diagnostic criteria for neuromyelitis optica
spectrum disorders
Background
The term ‘neuromyelitis optica’ (NMO) was coined in
1894 and has since been used to refer to the simultan-
eous or successive occurrence of optic nerve and spinal
cord inflammation [1]. In the majority of cases, the
syndrome is caused by autoantibodies to aquaporin-4,
the most common water channel in the central nervous
system (AQP4-IgG) [2–5]. Howe ver, 10-20% of patients
with NM O are negative for AQP 4-IgG [6–9]. Recent
studies by us and others have demonstrated the presence
of IgG antibodies to myelin oligodendrocyte glycopro-
tein (MOG-IgG) in a subset of patients with NMO as
well as in patients with isolated ON or longitudinally ex-
tensive transverse myelitis (LETM), syndromes that are
often formes frustes of NMO [10–12].
Most studies to date have found MOG-IgG exclusively
in AQP4-IgG-negative patients [11– 17]. Moreover, the
histopathology of brain and spinal cord lesions of MOG-
IgG-positive patients has been shown to differ from that
of AQP4-IgG-posititve patients [18–20]. Finally, evi-
dence from immunological studies suggests a direct
pathogenic role of MOG-IgG both in vitro and in vivo
[10, 21]. Accordingly, MOG-IgG-related NMO is now
considered by many as a disease entity in its own right,
immunopathogenetically distinct from its AQP4-IgG-
positive counterpart. However, the cohorts included in
previous clinical studies were relatively small (median 9
patients in [10–17, 22–24]) and the observation periods
often short (median 24 months in [11–13, 15–17, 23–26]).
Moreover, some previous studies did not, or not predom-
inantly, include Caucasian patients [12, 15, 26], which is
potentially important since genetic factors are thought to
play a role in NMO [27].
In the present study, we syste matically e valuated the
clinical and paraclinical features of a large cohort of 50
almost exclusively Caucasian patients with MOG-IgG-
positive optic neuritis (ON) and/or LETM. We report
on (i) epidemiological features; (ii) clinical presentation
Jarius et al. Journal of Neuroinflammation (2016) 13:280 Page 2 of 45

at onset; (iii) disease course; (iv) time to second attack;
(v) type and frequency of clinical attacks; (vi) brain, optic
nerve, and spinal cord magnetic resonance imaging
(MRI) features; (vii) cerebrospinal fluid (CSF) findings;
(viii) electrophysiological features (VEP, SSEP); (ix) type
and frequency of coexisting autoimmunity; (x) type and
frequency of preceding infections; (xi) association with
neoplasms; (xii) association with pregnancy and delivery;
(xiii) treatment and outcome of acute attacks; (xiv) re-
sponse to long-term treatments; and (xv) the long-term
prognosis. In addition, we evaluated whether and how
many MOG-IgG-positive patients with ON and/or mye-
litis met Wingerchuk’s revised 2006 diagnostic criteria
for NMO [28], the new 2015 international diagnostic
consensus criteria for NMO spectrum disorders
(NMOSD) [29], Barkhof’s MRI criteria for MS, and/or
McDonald’s clinicoradiological criteria for MS.
The present study forms part of a series of articles on
MOG-IgG in NMO and related disorders. In part 1, we
investigated the frequency and syndrome specificity of
MOG-IgG among patients with ON and/or LETM,
reported on MOG-IgG titers in the long-term course of
disease, and analyzed the origin of CSF MOG-IgG [30]. In
part 3, we describe in detail the clinical course and presen-
tation of a subgroup of patients with brainstem encephal-
itis and MOG-IgG-associated ON and/or LETM, a so far
under-recognized manifestation of MOG-related auto-
immunity [31]. Part 4 is dedicated to the visual system
in MOG-IgG-positive patient s with ON and reports
findings from optical coherence tomography (OC T ) in
this entity [32].
Methods
Clinical and paraclinical data of 50 MOG-IgG-positive
patients from 12 non-pediatric academic centers were
retrospectively evaluated; eight of the participating cen-
ters are members of the German Neuromyelitis optica
Study Group (NEMOS) [33–37]. MOG-IgG was de-
tected using an in-house cell-based assay (CBA) employ-
ing HEK293A cells transfected with full-length human
MOG as previously described [10] and confirmed by
means of a commercial fixed-cell ba sed assay employing
HEK293 cells transfected with full-le ngth human MOG
(Euroimmun, Lübeck, Germany) (see part 1 of this art-
icle series for details [30]). The study was approved by the
institutional review boards of the participating centers, and
patients gave written informed consent. Averages are given
as median and range or mean and standard deviation as in-
dicated. Fisher’s exact test was used to compare frequencies
between groups and the Mann-Whitney U test to compare
medians between groups. Due to the exploratory nature of
this study no Bonferroni correction was performed. P
values <0.05 were considered statistically significant.
Case reports
As reliable ce ll-based assays for t he dete ction of MOG-
IgG have become available only re cently, large and
comprehensive case series illustrating the broad and
heterogeneous spectrum of clinical manifestations, disease
courses, and radiological presentations are lacking so far.
We therefore decided to present, in addition to descriptive
statistical data, detailed reports on all cases evaluated in
order to draw for the first time a more vivid ‘real-life’ pic-
ture of this rare disorder than statistical analyses alone
could provide. Moreover, only detailed case descriptions
allow evaluation of treatment responses and outcomes in
a meaningful way in a retrospective setting. This is im-
portant, since randomized treatment trials in MOG-IgG-
positive ON or myelitis do not exist so far and will not be
performed in the near future due to the rarity of the con-
dition. The reports are to be found in the Appendix of this
paper and in the Case reports section in part 3 of this
article series [31].
Results
Epidemiological findings
Thirty-seven of the 50 MOG-IgG-positive patients were
female, corresponding to a sex ratio of 1:2.8 (m:f) (Fig. 1a).
Median age at onset was 31 years (35.5 years in patients
presenting with isolated ON [N = 32] and 28.5 years in the
remainder [N =18]; p < 0.04) with a broad range of 6 to
70 years. 3 patients were > =60 years of age at onset, and 8
patients were under 18 at first attack (including 4 ≤
12 years) (Fig. 1b). Fourty-nine of the 50 patients (98%)
were of Caucasian and 1 of Asian descent. Symptoms had
started between Jul 1973 and Apr 2016. The mean obser-
vation period since disease onset was 75 ± 46.5 months
(range 1-507 months). In line with the fact that many
MOG-IgG-positive patients develop ON and myelitis only
successively, the mean observation period was longer in
patients with a history both of ON and of myelitis at last
follow-up (88.6 months; N = 22) than in patients with a
history of either ON but no myelitis or myelitis but not
ON (64.6 months; N =28).
Disease course
Fourty of 50 MOG-IgG-positive patients (80%) had a
relapsing disease course. In the remaining 10 cases only
a single attack had occurred at last follow-up. The pro-
portion of patients with a monophasic course declined
with increasing observation time (Fig. 2, upper panel). If
only patients with a very long observation period
(≥8 years) are considered, 93% (13/14) had a recurrent
course (Fig. 2, lower panel). In line with this finding,
the median observati on time was shorter in the ‘mono-
phasic ’ than in the relapsing cases (26 vs. 52.5 months).
The proportion of patients with a relapsing disease
Jarius et al. Journal of Neuroinflammation (2016) 13:280 Page 3 of 45
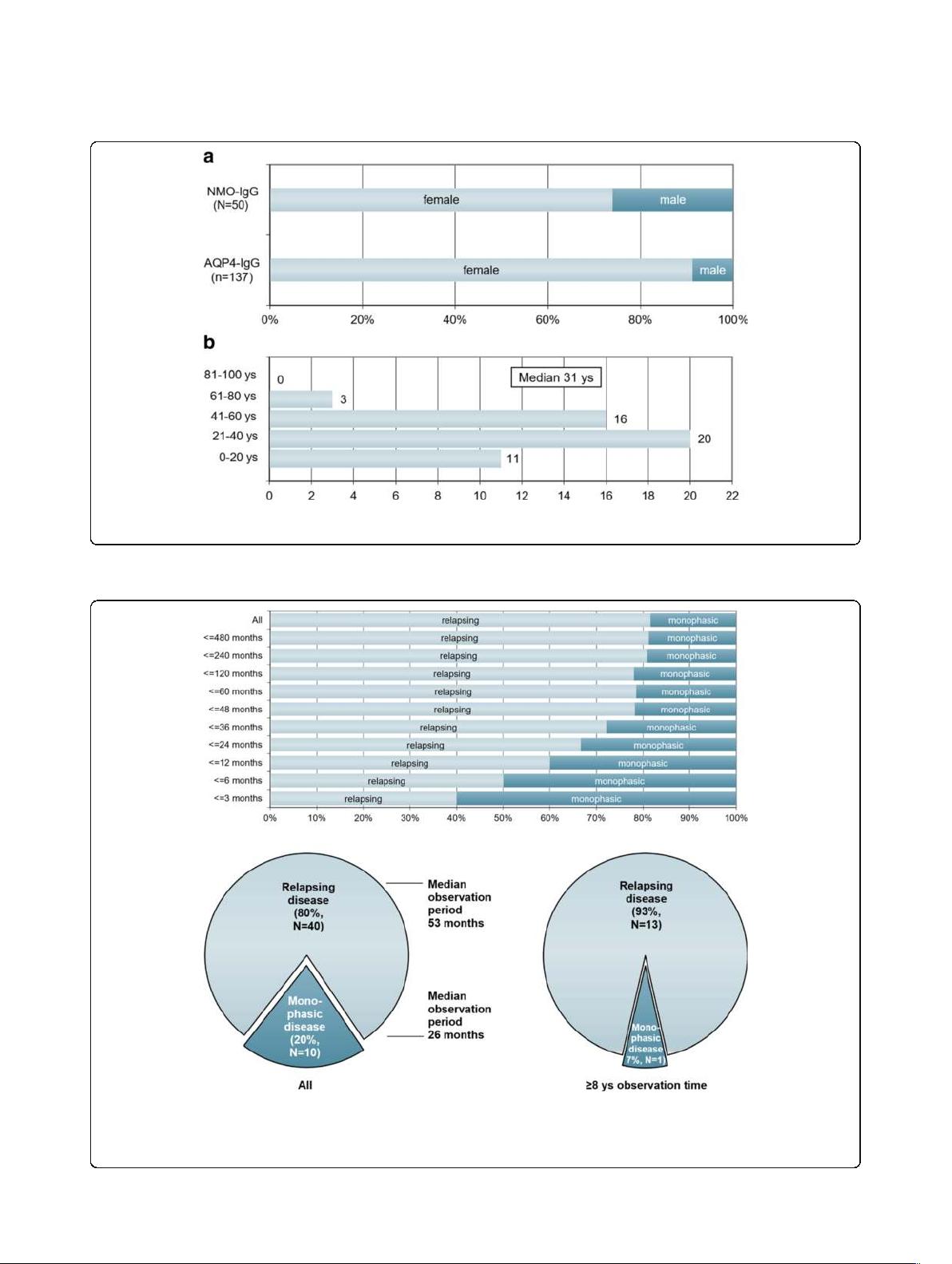
Fig. 1 Sex ratio and age distribution. a Sex ratio in MOG-IgG-positive patients with ON and/or LETM compared with AQP4-IgG-positive ON and/or LETM
(the latter data are taken from ref. [34]). b Age distribution at disease onset in 50 MOG-IgG-positive patients with ON and/or myelitis
Fig. 2 Disease course in relation to observation time in 50 MOG-IgG-positive patients with ON and/or myelitis. Upper panel: Note the decrease in
the proportion of monophasic cases with increasing observation time; however, in some patients no relapse has occurred more than 10 years
after the initial attack. Lower panel: Note the shorter observation time in the ‘monophasic’ group (left lower panel) and the lower percentage of
non-relapsing cases among patients with a long observation period ( ≥8 years; right lower panel)
Jarius et al. Journal of Neuroinflammation (2016) 13:280 Page 4 of 45

course did not differ significantly between female (83.8%
[31/37]) and male (69.2% [9/13]) patients.
Symptoms developed acutely or subacutely in the vast
majority of cases; progressive deterioration of symptoms
was very rare (at least once in 3/46 or 7%) and reported
only in patients with mye litis.
Clinical presentation during acute attacks
Overall, 276 clinically apparent attacks in 50 patients were
documented. 205 attacks clinically affected the optic nerve,
73 the spinal cord, 20 the brainstem, 3 the cerebellum, and
9 the supratentorial brain. 44/50 (88%) patients developed
at least once acute ON, 28/50 (56%) at least once acute
myelitis, 12/50 (24%) at least once a brainstem attack, 2/50
(4%) acute cerebellitis, and 7/50 (14%) acute supratentorial
encephalitis (Fig. 3, upper panel).
At la st follow-up, 26/50 (52%) patients had develope d
at least two different clinical syndromes (i.e., combina-
tions of ON, myelitis, brainstem encephalitis, cerebellitis,
and/or supratentorial encephalitis), either simultaneously
or successively. Of these, 22 (84.6%) had experienced at-
tacks both of ON and of myelitis at last follow-up (cor-
responding to 44% [22/50] of the total cohort). Another
22 (44%) had a history of ON but not of myelitis (recur-
rent in 15 or 68.2%), and 6 (12%) had a history of mye-
litis but not ON (recurrent in 4; LETM in all) at last
follow-up (Fig. 3, lower panel).
Myelitis and ON had occurred simultaneously (with and
without additional brainstem or brain involvement) at least
once in 9/22 (40.9%) patients with a history of both ON
and myelitis at last follow-up (and in 18% or 9/50 in the
total cohort).
Overall, 16/50 (32%) patients presented at least once
with more than one syndrome during a single attack
(more than once in 10/16). While 15 attacks of myelitis
(without ON) in 11 patients were associated with clinical
signs and symptoms of simultaneous brain or brainstem
involvement, only 1 attack of ON (without myelitis)
in 1 patient had this a ssociation. Clinically inapparent
spinal cord, brain, o r brainstem involvement was
detected in further patients by MRI (see Brain MRI
findings below and part 3 of this article series [31]
for details).
Symptoms associated with acute myelitis
Symptoms present at least once during attacks of myeli-
tis included tetraparesis in 8/29 (27.6%) patients, para-
paresis in 14/29 (48.3%), hemiparesis in 2/29 (6. 9%),
and monoparesis in 2/29 (6.9%). Paresis was severe
(BMRC grades ≤2) at lea st o nce in 6/29 (20.7 %)
patients. Attacks included at least once pain and
dysesthesia in 19/28 (67.9%) patients and were purely
sensory in 15/29 (51.7%). Sensory symptoms included
also Lhermitte’s sign. Bladder a nd/or bowel and/or
Fig. 3 Attack history at last follow-up. Upper panel: Frequencies of MOG-IgG-positive patients (N = 50) with a history of clinically manifest acute
optic neuritis (ON), myelitis (MY), brainstem encephalitis (BST), supratentorial encephalitis (BRAIN), and cerebellitis (CBLL) at last follow-up. Lower
panel: Frequencies of MOG-IgG patients with a history of optic neuritis (ON) and myelitis, ON but not myelitis, and myelitis (LETM in all cases) but
not ON, respectively, at last follow-up (n = 50)
Jarius et al. Journal of Neuroinflammation (2016) 13:280 Page 5 of 45