HAL Id: hal-00327858
https://hal.archives-ouvertes.fr/hal-00327858
Submitted on 8 Mar 2004
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-
entic research documents, whether they are pub-
lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diusion de documents
scientiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
3-D chemistry-transport model Polair: numerical issues,
validation and automatic-dierentiation strategy
Vivien Mallet, B. Sportisse
To cite this version:
Vivien Mallet, B. Sportisse. 3-D chemistry-transport model Polair: numerical issues, validation and
automatic-dierentiation strategy. Atmospheric Chemistry and Physics Discussions, European Geo-
sciences Union, 2004, 4 (2), pp.1371-1392. �hal-00327858�

ACPD
4, 1371–1392, 2004
3-D
chemistry-transport
model Polair
V. Mallet and B. Sportisse
Title Page
Abstract Introduction
Conclusions References
Tables Figures
J I
J I
Back Close
Full Screen / Esc
Print Version
Interactive Discussion
© EGU 2004
Atmos. Chem. Phys. Discuss., 4, 1371–1392, 2004
www.atmos-chem-phys.org/acpd/4/1371/
SRef-ID: 1680-7375/acpd/2004-4-1371
© European Geosciences Union 2004
Atmospheric
Chemistry
and Physics
Discussions
3-D chemistry-transport model Polair:
numerical issues, validation and
automatic-differentiation strategy
V. Mallet and B. Sportisse
CEREA, Joint Research Laboratory,
´
Ecole Nationale des Ponts et Chauss
´
ees, EDF R&D,
France
Received: 3 October 2003 – Accepted: 3 March 2004 – Published: 8 March 2004
Correspondence to: V. Mallet (mallet@cerea.enpc.fr)
1371

ACPD
4, 1371–1392, 2004
3-D
chemistry-transport
model Polair
V. Mallet and B. Sportisse
Title Page
Abstract Introduction
Conclusions References
Tables Figures
J I
J I
Back Close
Full Screen / Esc
Print Version
Interactive Discussion
© EGU 2004
Abstract
We briefly present in this short paper some issues related to the development and the
validation of the three-dimensional chemistry-transport model Polair.
Numerical studies have been performed in order to let Polair be an efficient and
robust solver. This paper summarizes and comments choices that were made in this5
respect.
Simulations of relevant photochemical episodes were led to assess the validity of
the model. The results can be considered as a validation, which allows next studies to
focus on fine modeling issues.
A major feature of Polair is the availability of a tangent linear mode and an adjoint10
mode entirely generated by automatic differentiation. Tangent linear and adjoint modes
grant the opportunity to perform detailed sensitivity analyses and data assimilation.
This paper shows how inverse modeling is achieved with Polair.
1. Introduction
Several 3-D chemistry-transport models (hereafter CTM) are now available and have15
proven to be efficient in many applications from passive-transport simulations to data
assimilation with highly non-linear models. One may cite Cmaq (Byun et al., 1998),
Chimere (Vautard et al., 2001), Eurad (Hass et al., 1995), etc. However, building a
code flexible enough to be able to deal with any of those applications still remains a
challenge.20
Polair is an Eulerian three-dimensional chemistry-transport model developed at
ENPC (
´
Ecole Nationale des Ponts et Chauss
´
ees). It is designed to handle a wide
range of applications. Thus it has been used for passive transport by Issartel et al.
(2003), for impact at European scale, for photochemistry by Sartelet et al. (2002) and
mercury chemistry by Roustan et al. (2003). Developments are led to include size25
resolved aerosols and other chemical mechanisms. In this paper, we focus on photo-
1372

ACPD
4, 1371–1392, 2004
3-D
chemistry-transport
model Polair
V. Mallet and B. Sportisse
Title Page
Abstract Introduction
Conclusions References
Tables Figures
J I
J I
Back Close
Full Screen / Esc
Print Version
Interactive Discussion
© EGU 2004
chemistry.
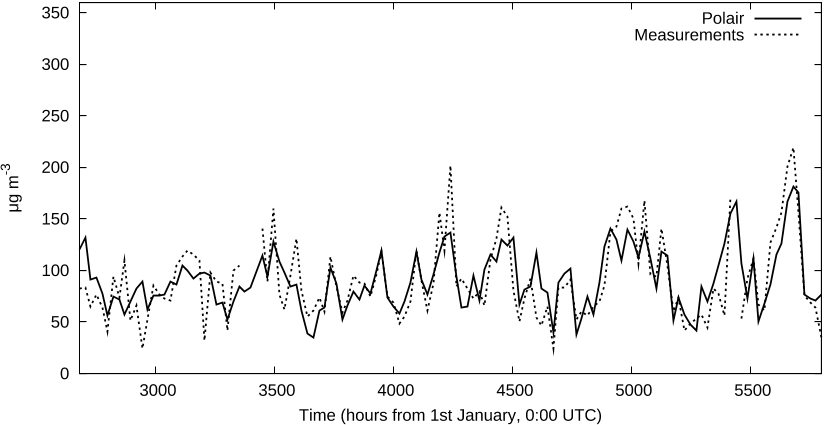
Beyond academic simulations coming along with code validation and basic modeling
work, validations were undertaken through comparisons with other models and with
measurements provided by dedicated campaigns. Therefore fine analyses identified
strengths and weaknesses of the model. Some analyses may be found in papers5
previously cited. This paper reports results obtained at regional scale and European
scale. At regional scale, the intensive obser vation period (hereafter IOP) # 2 of the
ESQUIF campaign (from summer 1998 to winter 2001, over
ˆ
Ile de France) enables
comparisons with measurements. At European scale, a simulation over four months
(from May to August 2001) was compared to measurements from several countries.10
A major advantage of Polair is the tangent linear mode and the adjoint mode that are
entirely generated by automatic differentiation. The availability of those modes enables
to get sensitivities with respect to most parameters and to put into practice virtually any
data-assimilation method involving the derivative of some output variables with respect
to some input parameters. In this paper, we explain the whole process.15
The paper is organized as follows. We first describe some abilities of Polair and we
point out some numerical issues. Then we report results from validations against mea-
surements from the ESQUIF campaign (regional scale) and measurements collected
for four months in 2001 over Europe. Finally we explain the way the tangent linear and
adjoint codes are generated and used.20
2. Model overview
As an Eulerian three-dimensional chemistry-transport model, Polair handles basic
physical processes by integrating in time the following equation:
∂c
i
∂t
+ div
(
c
i
· V
)
= div
(
K · ∇c
i
)
+ χ
i
(c) − D
i
+ E
i
(1)
where i labels a chemical species, c is a vector of chemical concentrations, V is the25
wind vector, K is the diffusion matrix, χ
i
combines production and loss terms of chemi-
1373

ACPD
4, 1371–1392, 2004
3-D
chemistry-transport
model Polair
V. Mallet and B. Sportisse
Title Page
Abstract Introduction
Conclusions References
Tables Figures
J I
J I
Back Close
Full Screen / Esc
Print Version
Interactive Discussion
© EGU 2004
cal reactions, D
i
is the deposition term (dry deposition and wet deposition – scaveng-
ing), E
i
stands for the emissions (surface and volumic emissions).
In our model, V is prescribed (e.g. by offline meteorological forecasts). The diffusion
matrix is assumed to be a diagonal matrix. Horizontal diffusion coefficients K
xx
and
K
yy
are not well known and are assumed constant in time and space. The vertical5
diffusion coefficient is estimated according to a given parameterization. For instance,
we usually use the parameterization proposed in Louis (1979).
Chemical reactions are modeled by χ
i
which depends on species concentrations.
For the time being, Polair has several chemical mechanisms:
– Mercury chemistry: simplified mechanism based on Petersen (1995);10
– Aerosols: modal approximation for inorganic aerosols;
– Photochemistry (for ozone): several mechanisms are available among which
RADM 2 (Stockwell et al., 1990), RACM (Stockwell et al., 1997), EURORADM
(Schell, 2000), MOCA (Aumont, 1994), CBM IV (Gery et al., 1989).
Dry gaseous deposition D
i
is computed as in Wesely (1989) or as in Baer et al.15
(1992), and required land use coverage data may be provided by the USGS data
base (http://edcdaac.usgs.gov/glcc/glcc.html). Wet deposition is parameterized as in
Sportisse et al. (2002).
Emissions E
i
are generated from coarse emissions of a few emitting classes and for
a few aggregated species. Speciation and aggregation stages are followed to estimate20
E
i
as in Middleton et al. (1990).
Equation (1) is relevant at many scales, which lets a CTM be relevant from regional
scale to continental scale. Since Polair can easily handle several chemical mecha-
nisms, different applications may reasonably be led at those scales.
The modularity of Polair has been strengthened by computing all parameteriza-25
tions in preprocessed steps. As such, Polair only is a numerical platform for solving
1374