1
Safety and Immunogenicity of Inactivated SARS-CoV-2
Vaccine in High-Risk Occupational Population: a
randomized, parallel, controlled clinical trial
Yongliang Feng
a,b#
, Jing Chen
c,d#
, Tian Yao
a,b#
,
Yue Chang
a,b
, Xiaoqing Li
c,d
, Rongqin
Xing
e
, Hong Li
c,d
, Ruixue Xie
a,b
, Xiaohong Zhang
c,d
, Zhiyun Wei
c,d
, Shengcai Mu
c,d
,
Ling Liu
c,d
, Lizhong Feng
c,d
* & Suping Wang
a,b
*
a
School of Public Health, Shanxi Medical University, Taiyuan, China
b
Center of Clinical Epidemiology and Evidence Based Medicine, Shanxi Medical
University, Taiyuan, China
c
Shanxi Provincial Center for Disease Control and Prevention, Taiyuan, China
d
Shanxi Provincial Key Laboratory for major infectious disease response
e
Outpatient Department of Shanxi Aviation Industry Group Co. LTD
#
Co-first authors
*Author for correspondence:
Suping Wang, professor, Email: supingwang@sxmu.edu.cn; Address: Department of
Epidemiology, School of Public Health, Shanxi Medical University, 56 Xinjian South
Road Taiyuan, 030001, Shanxi Province, China
Lizhong Feng, chief physician, Email: 1508717672@qq.com; Address: Shanxi
Provincial Center for Disease Control and Prevention, 8 Xiaonanguan Street Taiyuan,
030012, Shanxi Province, China
. CC-BY-NC-ND 4.0 International licenseIt is made available under a
is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. (which was not certified by peer review)
The copyright holder for this preprint this version posted August 9, 2021. ; https://doi.org/10.1101/2021.08.06.21261696doi: medRxiv preprint
NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice.

2
Abstract
Vaccination is urgently needed to prevent the global spread of severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2). Here, we conducted a
randomized, parallel, controlled clinical trial for assessment of the immunogenicity
and safety of an inactivated SARS-CoV-2 vaccine, aiming to determine an appropriate
vaccination interval for high-risk occupational population. Participants were randomly
assigned to receive two doses of inactivated SARS-CoV-2 vaccine (4 µg per dose) at
an interval of either 14 days, 21 days or 28 days. The primary immunogenicity
endpoints were neutralization antibody seroconversion and geometric mean titer
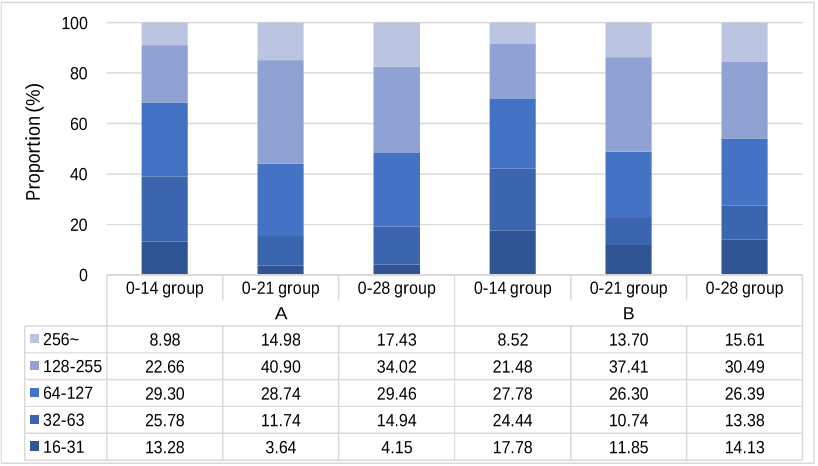
(GMT) at 28 days after the second dose. Our results showed that the seroconversion
rates (GMT
≥
16) were all 100% in the three groups and the 0-21 and 0-28 groups
elicited significantly higher SARS-CoV-2 neutralizing antibody level. All reported
adverse reactions were mild. (Chinese Clinical Trial Registry: ChiCTR2100041705,
ChiCTR2100041706)
Introduction
The ongoing pandemic of coronavirus disease 2019 (COVID-19) induced by
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to an
unprecedented global public health crisis. Globally, as of August 4, 2021, more than
199 million cases of SARS-CoV-2 infection and more than 4.2 million deaths have
been reported
1
. SARS-CoV-2 belongs to the Betacoronavirus of the family
Coronaviridae, and commonly induces a spectrum of clinical manifestations ranging
from asymptomatic, minor flu-like symptoms to acute respiratory distress syndrome
(ARDS), pneumonia and even death
2
. Compared with other coronaviruses,
SARS-CoV-2 appears to undergo more rapid transmission and variation
3, 4
. Although
it is proved to be effective that the COVID-19 pandemic can be controlled using strict
social hygiene measures such as physical distancing and masks, the absence of herd
immunity leaving people susceptible to further waves of SARS-CoV-2 infection,
. CC-BY-NC-ND 4.0 International licenseIt is made available under a
is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. (which was not certified by peer review)
The copyright holder for this preprint this version posted August 9, 2021. ; https://doi.org/10.1101/2021.08.06.21261696doi: medRxiv preprint

3
especially for the high-risk occupational population. Meantime, the measures taken to
contain SARS-CoV-2 have placed a substantial burden on health-care systems around
the world, with far-reaching social and economic consequences. Hence, a safe and
effective vaccine against COVID-19 is urgently needed to prevent the resurgence of
the epidemic.
Many countries have accelerated the process of clinical trials to determine an
effective and safe vaccine to prevent COVID-19 pandemic. Currently, more than 292
candidate vaccines are in development worldwide, 37 of which are already in phase 3
trials using different platforms
5
, including nucleic acid (DNA and RNA) vaccines,
viral vector (replicating and non-replicating) vaccines, virus-like particles vaccines,
peptide-based vaccines, recombinant protein vaccines and inactivated vaccines
6-8
.
Inactivated vaccines have been widely used against various infectious diseases for
decades. Their long history of use confers some advantages, such as well-developed
and mature manufacturing processes, ease of scaling up production and storage, and
the ability to present multiple viral proteins for immune recognition. In addition,
inactivated vaccines induce high levels of neutralizing antibody titers in mice, rats,
guinea pigs, rabbits, and nonhuman primates to provide protection against
SARS-CoV-2
9-11
. Moreover, the results of previous clinical trials on the inactivated
vaccines conducted in several countries showed good neutralizing antibody responses
and/or efficacy against disease caused by COVID-19
12-21
. To date, two inactivated
SARS-CoV-2 vaccines manufactured by the Beijing Institute of Biological
Products/Sinopharm (China) and Sinovac Life Sciences/CoronaVac (China) have been
placed on WHO's Emergency Use Listing. Here, we report the analysis of
immunogenicity and safety of an inactivated SARS-CoV-2 vaccine manufactured by
Beijing Institute of Biological Products Co., Ltd (China).
Previous studies
15-17, 22-24
have shown that the three immunization programs (0,
14 procedure, 0, 21 procedure or 0, 28 procedure) induce varying degrees of immune
effect, but the optimal interval of injections remains unclear. Furthermore, the safety
and immunogenicity of inactivated SARS-CoV-2 vaccine in occupational high-risk
. CC-BY-NC-ND 4.0 International licenseIt is made available under a
is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. (which was not certified by peer review)
The copyright holder for this preprint this version posted August 9, 2021. ; https://doi.org/10.1101/2021.08.06.21261696doi: medRxiv preprint

4
population have not been reported. Therefore, based on the preliminary clinical trials,
we explored the immunogenicity and safety of the three different SARS-CoV-2
inactivated vaccination schemes at an interval of either 14 days, 21 days or 28 days in
high-risk occupational population to optimize the inactivated vaccination regimen. We
would continue to follow up until months 3, 6, and 12 in the further study.
Methods
Study design and participants
We conducted a randomized, controlled clinical trial of the SARS-CoV-2
inactivated vaccine manufactured by Beijing Biological Products Institute Co., Ltd. in
Taiyuan City, Shanxi Province, China. Written informed consents were obtained from
all participants before enrollment. Eligible participants were people aged 18-59 years,
signed the informed consent form and participated voluntarily with good compliance.
Exclusion criteria were participants with history or family history of allergy,
convulsion, epilepsy, encephalopathy or psychosis; any intolerance or allergy to any
component of the vaccine; known or suspected diseases including severe respiratory
disease, severe cardiovascular disease, severe liver or kidney disease, medically
uncontrollable hypertension (systolic blood pressure
≥
140 mmHg and diastolic blood
pressure
≥
90 mmHg), complications of diabetes mellitus, malignancy, various acute
diseases or acute episodes of chronic disease; various infectious, suppurative and
allergic skin diseases; congenital or acquired immunodeficiency, other vaccination
history within 14 days before vaccination, a history of coagulation dysfunction, a
history of non-specific immunoglobulin injection within 1 month prior to enrollment,
acute illness with fever (body temperature > 37.0°C); and being pregnant or
breastfeeding.
The protocol was approved by the Ethics Committee of Shanxi Provincial Center
for Disease Control and Prevention and was conducted in accordance with the
Declaration of Helsinki and Good Clinical Practice. All participants signed a consent
. CC-BY-NC-ND 4.0 International licenseIt is made available under a
is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. (which was not certified by peer review)
The copyright holder for this preprint this version posted August 9, 2021. ; https://doi.org/10.1101/2021.08.06.21261696doi: medRxiv preprint

5
form after being informed about the study. The trial was registered with
ChiCTR.org.cn (ChiCTR2100041705, ChiCTR2100041706).
Procedures
A computerized random number generator performed block randomization with
a randomly selected block size of 6, and eligible participants were randomly assigned
into three groups to receive two doses inactivated COVID-19 vaccine at the schedule
of day 0-14, day 0-21, or day 0-28. Each dose of vaccine containing 4 µg of
inactivated SARS-CoV-2 virus antigen was intramuscularly injected into the lateral
deltoid muscle of the upper arm. The vaccines used in this study were inactivated
vaccine (Vero Cell) produced by Beijing Biological Products Institute Co., Ltd.
Demographic information (age, gender, body mass index (BMI), marital status, and
education level), influenza vaccination history, smoking, drinking, and chronic
diseases were collected via questionnaire investigation.
Safety assessment
After each dose was vaccinated, the participants were observed for any
immediate reaction for 30 min, and local and systemic adverse reactions were
collected. Participants were required to record the local adverse events and systemic
adverse events on diary cards within 7 days of each injection. Any other unsolicited
symptoms were also recorded during a 28-day follow-up period after each injection
by spontaneous report from the participants combined with the regular visit. The
solicited adverse reactions included local reactions (pain, induration, swelling, rash,
flush, and pruritus) and systematic reactions (fever, diarrhea, dysphagia, anorexia,
vomiting, nausea, muscle pain (non-vaccination sites), arthralgia, headache, cough,
dyspnea, skin and mucosal abnormalities, acute allergic reactions, and fatigue).
Laboratory methods
Oropharyngeal/nasal swabs were collected for detecting SARS-CoV-2 nucleic
. CC-BY-NC-ND 4.0 International licenseIt is made available under a
is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. (which was not certified by peer review)
The copyright holder for this preprint this version posted August 9, 2021. ; https://doi.org/10.1101/2021.08.06.21261696doi: medRxiv preprint