1
A novel antiviral lncRNA EDAL shields a T309 O-GlcNAcylation site to 1
promote EZH2 degradation 2
Baokun Sui
1,2,
*, Dong Chen
3,4,
*, Wei Liu
1,2
, Qiong Wu
1,2
, Bin Tian
1,2
, Jing 3
Hou
3,4
, Yingying Li
1,2
, Shiyong Liu
5
, Juan Xie
5
, Hao Jiang
6
, Zhaochen Luo
1,2
, 4
Lei Lv
1,2
, Fei Huang
1,2
, Ruiming Li
1,2
, Min Cui
1,2
, Ming Zhou
1,2
, Huanchun 5
Chen
1,2
, Zhen F. Fu
1,2,7
, Yi Zhang
3,4,
†
, Ling Zhao
1,2,
†
6
Running title: lncRNA EDAL inhibits neurotropic viruses 7
1
State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, 8
Wuhan, 430070, China; 9
2
Key Laboratory of Preventive Veterinary Medicine of Hubei Province, College of 10
Veterinary Medicine, Huazhong Agricultural University, Wuhan, 430070, China; 11
3
Center for Genome analysis and
4
Laboratory for Genome Regulation and Human Health, 12
ABLife Inc., Wuhan, 430075, China 13
5
School of Physics, Huazhong University of Science and Technology, Wuhan, 430074, 14
China; 15
6
Key Laboratory of Marine Drugs, Ministry of Education, School of Medicine and 16
Pharmacy, Shandong Provincial Key Laboratory of Glycoscience and Glycotechnology, 17
Ocean University of China, Qingdao, 266003, China; 18
7
Department of Pathology, University of Georgia, Athens, GA 30602, USA;
19
*
These authors contributed equally to this work. 20
certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprint (which was notthis version posted October 30, 2019. ; https://doi.org/10.1101/824813doi: bioRxiv preprint

2
†
To whom correspondence should be addressed: 21
Ling Zhao, Mailing address: State Key Laboratory of Agricultural Microbiology, Huazhong 22
Agricultural University, Wuhan, 430070, China.Tel: +86-27-8728 5016; Fax: +86-27-8728 23
2608; E-mail: zling604@yahoo.com 24
Correspondence may also be addressed to 25
Yi Zhang, Mailing address: Center for Genome analysis and Laboratory for Genome 26
Regulation and Human Health, ABLife Inc., Wuhan, 430075, China. E-mail: 27
yizhang@ablife.cc
28
29
certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprint (which was notthis version posted October 30, 2019. ; https://doi.org/10.1101/824813doi: bioRxiv preprint

3
Abstract 30
The central nervous system (CNS) is vulnerable for viral infection, yet few host 31
factors in the CNS are known to defend invasion by neurotropic viruses. We 32
report here that multiple neurotropic viruses, including rabies virus (RABV), 33
vesicular stomatitis virus (VSV), Semliki Forest virus (SFV) and herpes 34
simplex virus 1 (HSV-1), elicit the neuronal expression of a host-encoded 35
lncRNA EDAL. EDAL inhibits the replication of these neurotropic viruses in 36
neuronal cells and RABV infection in mouse brains. EDAL binds to the 37
conserved histone methyltransferase enhancer of zest homolog 2 (EZH2) and 38
specifically causes EZH2 degradation via lysosomes, reducing the cellular 39
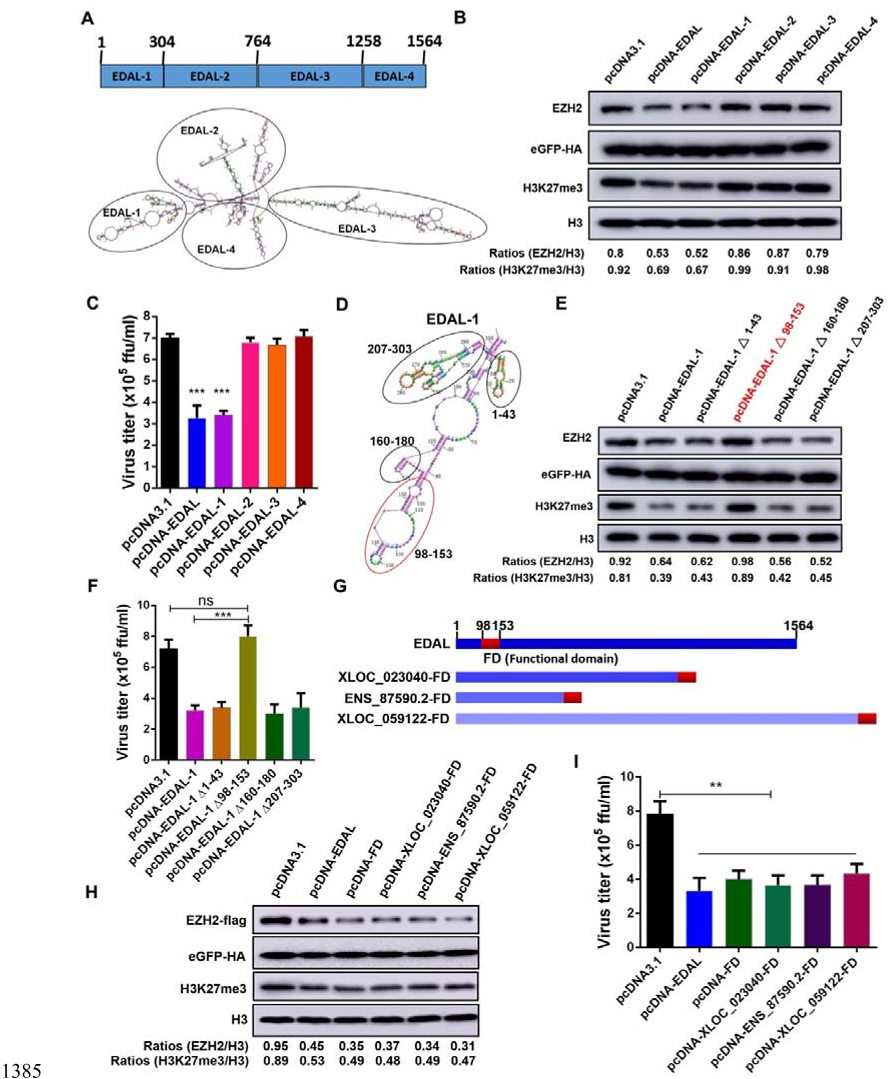
H3K27me3 level. The antiviral function of EDAL resides in a 56-nt antiviral 40
substructure through which its 18-nt helix-loop intimately contacts multiple 41
EZH2 sites surrounding T309, a known O-GlcNAcylation site. EDAL positively 42
regulate the transcription of Pcp4l1 encoding a 10 kDa peptide, which inhibits 43
the replication of mutiple neurotropic viruses. Our findings proposed a model in 44
which a neuronal lncRNA can exert an effective antiviral function via blocking a 45
specific O-GlcNAcylation that determines EZH2 lysosomal degradation. 46
Key words: EZH2/lncRNA/neurotropic virus/O-GlcNAcylation/PCP4L1 47
48
49
50
51
52
53
certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprint (which was notthis version posted October 30, 2019. ; https://doi.org/10.1101/824813doi: bioRxiv preprint

4
INTRODUCTION 54
Among infectious diseases of the central nervous system (CNS), those 55
caused by viral pathogens—known as neurotropic viruses—are far more 56
common than bacteria, fungi, and protozoans (2Nd & Mcgavern, 2015, Ludlow, 57
Kortekaas et al., 2016). Neurotropic viruses arrive to the CNS through multiple 58
routes and propagate within various cell types including astrocytes, microglia 59
and neurons, depending on the entering routes and virus types (Manglani & 60
McGavern, 2018). Infection of some neurotropic viruses can cause meningitis 61
or encephalitis and result in severe neurologic dysfunction, such as VSV, SFV, 62
HSV-1 and HIV etc. (Bradshaw & Venkatesan, 2016, Fragkoudis, 63
Dixon-Ballany et al., 2018, Gagnidze, Hajdarovic et al., 2016). Moreover, 64
nearly half of all emerging viruses are neurotropic viruses (Olival & Daszak, 65
2005), including the Dengue and Zika viruses (Carod-Artal, 2016, 66
Meyding-Lamade & Craemer, 2018). RABV is a typical neurotropic virus and is 67
the causative agent of rabies disease, a globally well-known and often lethal 68
encephalitis. Therefore, it is urgent to develop new approaches for therapies 69
as well as for cheaper and more effective vaccines against rabies (Fisher & 70
Schnell, 2018, Schnell, McGettigan et al., 2010). 71
Long non-coding RNAs (lncRNAs) are involved in the development, 72
plasticity, and pathology of the nervous system (Batista & Chang, 2013, Briggs, 73
Wolvetang et al., 2015, Fatica & Bozzoni, 2014, Sun, Yang et al., 2017). 74
Notably, around 40% of lncRNAs detected to date are expressed specifically in 75
the brain (Liu, Wang et al., 2017). Genome-wide association studies (GWASs) 76
and functional studies have associated lncRNAs with neurological diseases 77
including autism spectrum disorders (ASD), schizophrenia, Alzheimer’s 78
disease, and neuropathic pain, among others (Briggs et al., 2015). 79
Mechanistically, it has been shown that lncRNAs can regulate chromatin 80
modifications and gene expression, at both the transcriptional and the 81
certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprint (which was notthis version posted October 30, 2019. ; https://doi.org/10.1101/824813doi: bioRxiv preprint

5
post-transcriptional levels (Bonasio & Shiekhattar, 2014, Mercer, Dinger et al., 82
2009, Wang & Chang, 2011). LncRNAs have recently been shown to regulate 83
innate immune responses by either promoting or inhibiting viral genome 84
replication, highlighting them as a class of novel targets for developing antiviral 85
therapies (Carpenter & Fitzgerald, 2018, Fortes & Morris, 2016, Imamura, 86
Imamachi et al., 2014, Kambara, Niazi et al., 2014, Ma, Han et al., 2017, 87
Ouyang, Hu et al., 2016, Ouyang, Zhu et al., 2014). It is conceivable that 88
antiviral lncRNAs targeting none-innate immune response pathway may exist 89
in neuron cells and brains, which has not been documented yet. 90
Polycomb repressive complex 2 (PRC2) is a protein complex with an 91
epigenetic regulator function in maintaining the histone modifications that mark 92
transcriptional repression states which are established during early 93
developmental stages (Ringrose, 2017). Some lncRNAs are known to interact 94
with and direct PRC2 towards the chromatin sites of action, thusly defining a 95
trans-acting lncRNA mechanism (Jin, Lv et al., 2018, Rinn, Kertesz et al., 96
2007). The EZH2 methyltransferase enzyme is the catalytic component of 97
PRC2: it binds RNAs and catalyzes di- or tri-methylation of histone H3 lysine 98
27 (H3K27me2/3), a modification which leads to the formation of facultative 99
heterochromatin and thus to transcriptional repression (Justin, Zhang et al., 100
2016, Kasinath, Faini et al., 2018, Margueron & Reinberg, 2011). Many 101
cancers are known to feature very high EZH2 expression levels, so this protein 102
has emerged as an anticancer target for which multiple chemical inhibitors 103
have been developed (Kim & Roberts, 2016, Lee, Yu et al., 2018). It has also 104
been recently reported that inhibitors of the histone methyltransferase activity 105
of EZH2 can suppress infection by several viruses, suggesting a function of 106
EZH2 and/or PRC2 in regulating viral infection (Arbuckle, Gardina et al., 2017). 107
However, it is unclear how this regulation occurs. In general, PRC2 (EZH2) 108
binds different classes of RNAs in a promiscuous manner in vitro and in cells, 109
and some lncRNAs such as RepA RNA show in vitro specificity with PRC2 110
certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprint (which was notthis version posted October 30, 2019. ; https://doi.org/10.1101/824813doi: bioRxiv preprint