




read more










This review highlights the research aimed at the implementation of MOFs as an integral part of solid-state microelectronics. Both the fundamental and applied aspects of this two-pronged approach are discussed.
A low density of electrically active defects and high charge mobility are also essential for an efficient LED so that injected holes and electrons can penetrate the emitting layer(s) and recombine to form excitons withminimal non-radiative recombination.
MOFs are well suited to serve as the dye (i.e. light harvesting) component of a DSSC by virtue of the ability to build structures with multiple light absorbers locked into a stable crystalline structure.
The same material characteristics that make MOFs attractive for charge transport operate here, namely synthetic tunability, long-range order and porosity as an additional design element.
In AC circuit analysis, the generalized concept of impedance (Z, O) is defined in analogy to DC resistance, as the voltage to current ratio.
in some cases the MOF phase has an intrinsic preference for alignment parallel to the surface, because of the formation of well-defined crystal faces or the inherently layered nature of the material.
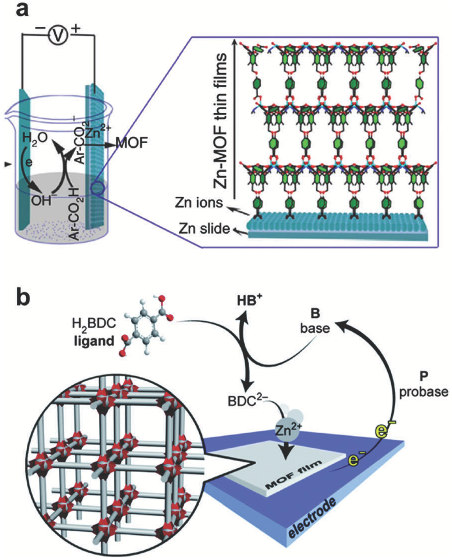
For instance, in a synthesis solution containing Zn(II) nitrate and terephthalic acid, the generation of OH ions through reduction of the NO3anion results in ligand deprotonation and formation of a MOF-5 film on the cathode.
The approach of using metal oxide films as precursors for MOF coatings benefits from a range of established technologies for deposition of the former (PVD, CVD, ALD, sol–gel methods, etc.).
While most of the proton-conducting frameworks require hydration, several promising strategies have been developed for anhydrous proton transport in MOFs.
replacing these systems with solid-state electrolytes is an active research topic, mainly because of their volatility, flammability and reactivity towards the electrodes.
In the context of microelectronic devices, integration of liquid components as part of vertical stacks is not possible due to structural reasons and incompatibility with vacuum technology.
Although nano-indentation is becoming a more common tool to characterize single MOF crystals and thin film, few materials have been characterized in depth.
To fabricate the first FETs based on semiconducting MOFs,28,46 interfacial growth processes were combined with subsequent film transfer.
Compared to established thin film deposition technologies (e.g. sol–gel methods, PVD, CVD, ALD), MOF films typically show a higher roughness, often above 10% of the film thickness.
In contrast to purely inorganic or organic conductors such as MoS2 or graphene, which do not easily lend themselves to chemical functionalization, the electronic properties of MOFs can be tuned by chemically altering the linker, metal ion or guest species adsorbed in the pore space.
recent studies demonstrated that analyte-permeable dielectric sensing materials can also be screened using the KP method.
It will be interesting to see the performance gain by combining MOFs with more recent sensors that exploit different elastic wave propagation modes including acoustic-plate-mode and flexural-plate-wave devices.