
Signaling Cross Talk between TGF-b/Smad
and Other Signaling Pathways
Kunxin Luo
Department of Molecular and Cell Biology, University of California, Berkeley, and Life Sciences
Division, Lawrence Berkeley National Laboratory, Berkeley, California 94720
Correspondence: kluo@berkeley.edu
Cytokines of the transforming growth factor b (TGF-b) family, including TGF-bs, bone mor-
phogenic proteins (BMPs), activins, and Nodal, play crucial roles in embryonic development
and adult tissue homeostasis by regulating cell proliferation, survival, and differentiation, as
well as stem-cell self-renewal and lineage-specific differentiation. Smad proteins are critical
downstream mediators of these signaling activities. In addition to regulating the transcription
of direct target genes of TGF-b, BMP, activin, or Nodal, Smad proteins also participate in
extensive cross talk with other signaling pathways, often in a cell-type- or developmental
stage-specific manner. These combinatorial signals often produce context-, time-, and loca-
tion-dependent biological outcomes that are critical for development. This review discusses
recent progress in our understanding of the cross talk between Smad proteins and signaling
pathways of Wnt, Notch, Hippo, Hedgehog (Hh), mitogen-activated protein (MAP), kinase,
phosphoinositide 3-kinase (PI3K)-Akt, nuclear factor kB (NF-kB), and Janus kinase/signal
transducers and activators of transcription (JAK/STAT) pathways.
T
he transforming growth factor b (TGF-b)
family of cytokines, including TGF-bs,
bone morphogenic proteins (BMPs), and acti-
vins, regulates a wide array of biological activi-
ties in various cell types and at different de-
velopmental stages. Smad proteins are critical
mediators of TGF-b, BMP, and activin signaling
(Feng and Derynck 2005; Heldin and Mousta-
kas 2011; Massague
´
2012). On phosphorylation
by the activated type-I receptor kinase, the re-
ceptor-associated R-Smads form a heteromeric
complex with the co-Smad and translocate into
the nucleus, where they interact with sequence-
specific DNA-binding cofactors and transcrip-
tional coactivators or corepressors to regulate
the transcription of target genes. Additionally,
the activity of this Smad pathway can be reg-
ulated by positive and negative modulators,
including the inhibitory Smads, Smad6 and
Smad7, the corepressors Ski and SnoN, and
the Smurf family of E3 ubiquitin ligases.
The Smad pathway is integrated into the
intracellular signaling network through cross
talk with other signaling pathways, and these
cross talk activities play important roles in the
regulation of various biological responses. The
cross talk can occur at multiple levels: by alter-
ing the expression and activities of ligands, an-
tagonists, receptors, and signaling components;
by incorporating into transcription complexes
Editors: Rik Derynck and Kohei Miyazono
Additional Perspectives on The Biology of the TGF-b Family available at www.cshperspectives.org
Copyright # 2016 Cold Spring Harbor Laboratory Press; all rights reserved
Advanced Online Article. Cite this article as Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a022137
1
on August 25, 2022 - Published by Cold Spring Harbor Laboratory Press http://cshperspectives.cshlp.org/Downloaded from

and/or inducing changes in chromatin modifi-
cation complexes that globally impact gene ex-
pression; and by direct interactions between
Smads and other intracellular signaling com-
ponents. This review discusses the cross talk of
Smads with Wnt, Notch, Hippo, Hedgehog
(Hh), mitogen-activated protein (MAP) kinase,
phosphoinositide 3-kinase (PI3K)-Akt, nuclear
factor kB(NF-kB), and JAK-STAT signaling
pathways, with a focus on the direct interactions
among key signaling components. This review
does not discuss the cross talk between TGF-
b-activated non-Smad signaling pathways and
other signaling pathways.
CROSS TALK WITH Wnt SIGNALING
The Wnt signaling pathways regulate many
aspects of vertebrate development and play
important roles in cell-fate determination,
self-renewal, and maintenance of stem and early
progenitor cells. Deregulation of Wnt signaling
is associated with various types of human can-
cer, including colorectal cancer and leukemia.
The canonical Wnt signaling pathway is initi -
ated on binding of a Wnt ligand to its cognate
receptor Frizzled and the transmembrane pro-
tein Lrp5 or Lrp6, and is primarily mediated by
b-catenin (Nusse 2012). In the absence of a Wnt
ligand, the newly synthesized b-catenin is found
in the destruction complex with the adenoma-
tous poly posis coli (APC) tumor suppressor
and scaffolding protein Axin, where it is phos-
phorylated by casein kinase I (CKI) and glyco-
gen synthase kinase-3b (GSK-3b) and targeted
for degradation. On ligand binding, Lrp5 or
Lrp6 binds to Axin in a Wnt- and phosphory-
lation-dependent manner, leading to the for-
mation of the complex containing Dishevelled
(Dvl), Axin, and GSK-3b. As a consequence,
the kinase activity of GSK-3b is inhibited, re-
sulting in stabilization of b-catenin. b-catenin
then translocates into the nucleus and binds
to the closely related T-cell factor (TCF) or lym-
phoid enhancer–binding factor (LEF) trans-
cription factors. With the help of additional
nuclear components, including BCL9, Pygopos,
and cAMP-response element-binding (CREB)-
binding protein (CBP), this binding converts
TCF or LEF from transcriptional repressors
into activators. Wnt signaling also regulates pla-
nar cell polarity through the noncanonical
pathway, by activating Rho and Rac signaling,
and modulates calcium release through G-pro-
tein-dependent activation of the phospholipase
C (PLC) pathway (Krausova and Korinek 2014).
Combinatorial TGF-b and Wnt Signaling
Is Essential for Early Development and
Tissue Homeostasis
Wnt signaling benefits from extensive cross
talk with other signaling pathways, particularly
TGF-b and BMP signaling, and the combinato-
rial signaling often occurs in early embryos to
allow overlapping signaling pathways to specify
different territories and cell fates. In early em-
bryos, extensive mutual regulation and cross talk
between Wnt and Nodal/activin/BMP path-
ways and later between Wnt and BMP signaling
exist at multiple levels, and these interactions are
essential for embryonic patterning and devel-
opment of multiple lineages. For example, in
Drosophila, the BMP ligand Decapentaplegic
(Dpp) and Wnt ligand Wingless (Wg) cooperate
to pattern the wings, legs, imaginal discs, brain
and midgut (Attisano and Labbe
´
2004). In Xe-
nopus, signals from both pathways are critical for
the establishment of Spemann’s organizer and
activation of many organizer-specific genes, in-
cluding those encoding Twin, Goosecoid, chor-
din, and Cerberus, as well as dorsal fate specifi-
cation in mesoderm and endoderm (Cui et al.
1996; Crease et al. 1998; Zorn et al. 1999; Labbe
´
et al. 2000; Nishita et al. 2000; Schohl and Fa-
gotto 2002; Xanthos et al. 2002). In zebrafish, the
two pathways together regulate posterior meso-
derm formation by synergistically activating the
expression of posterior mesoderm genes such as
tbx6 (Szeto and Kimelman 2004). In mouse em-
bryos, Wnt signaling modulates the expression
of the BMP target gene Msx2, either directly
or through induction of expression of BMP
ligands, thereby influencing cell fates in the
ectoderm and the neural crest (Hussein et al.
2003). In the dorsal telencephalon, Wnt and
BMP signaling regulate graded emx2 expression
in a cooperative manner (Theil et al. 2002).
K. Luo
2
Advanced Online Article. Cite this article as Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a022137
on August 25, 2022 - Published by Cold Spring Harbor Laboratory Press http://cshperspectives.cshlp.org/Downloaded from

In adult tissues, Wnt and BMP signaling
often interact to ensure proper tissue homeo-
stasis by regulating the expression of common
key target genes, and aberrant signal ing in either
pathwayoften contributes to carcinogenesis and
diseases. Compound heterozygote mice lacking
both Smad4 and APC develop more intestinal
or pancreatic tumors than deletion of APC
alone, and deletion of Smad2 accelerates colon
cancer progression in APC-deficient mice (Ta-
kaku et al. 1998; Cullingworth et al. 2002; Ha-
mamoto et al. 2002). However, a separate study
reported that compound Smad2/Apc heterozy-
gotes are indistinguishable from Apc-null mice
in intestinal tumor progression (Takaku et al.
2002), and argued that Smad4 plays a more
prominent role in coordinating with Wnt sig-
naling in the intestine. In support of these ob-
servations, TGF-b and Wnt were shown to syn-
ergize in the transcription activation of the Wnt
target gene encoding gastrin, a promoter of gas-
trointestinal cancer, indicating that TGF-b and
Wnt signaling can cooperate to promote tu-
morigenesis (Lei et al. 2004).
Mechanistically, the TGF-b/BMP and Wnt
pathways coordinate to regulate development
and homeostasis, likely by controlling the self-
renewal and differentiation of stem cells. In
mouse embr yonic stem (ES) cells (mESCs),
BMP, acting together with leukemia inhibitory
factor (LIF), maintains pluripotency and is es-
sential for self-renewal (Ying et al. 2003). In the
presence of both TGF-b and Wnt signaling,
however, BMP induces a posterior primitive-
streak (PS)-like fate and promotes differentia-
tion of PS-like cells into Flk1-expressing hema-
topoietic mesoderm (Nostro et al. 2008). In
the Flk1-expressing hematopoietic mesoderm,
BMP activates Wnt signaling, and the two sig-
nals then act together to activate the Cdx-Hox
pathway, leading to blood cell–fate commit-
ment (Lengerke et al. 2008). The presence of
TGF-b and Wnt signaling is required for the
initial inductive activity of BMP, because inhi-
bition of either of these signals abolishes the
inductive activity. Similarly, in human ES cells
(hESCs), BMP induces mesendoderm differen-
tiation together with fibroblast growth factor 2
(FGF2), and this activity requires TGF-b or
Wnt signaling (Yu et al. 2011). In early neural
crest stem cells, Wnt promotes sensory neuro-
genesis, whereas BMP antagonizes Wnt signal-
ing to suppress differentiation and neurogenesis
(Kleber et al. 2005). BMP also suppresses Wnt
signaling to maintain a proper balance in self-
renewal of intestinal stem cells in a phosphatase
and tensin homolog (PTEN)-Akt pathway-de-
pendent manner. BMP enhances the activity
of PTEN, leading to inactivation of Akt and
inhibition of the nuclear accumulation, and
transcription activity of b-catenin (He et al.
2004), resulting in inhibition of Wnt signaling.
Finally, in transformed mammary epithelial
cells, TGF-b and Wnt signaling synergize to in-
duce activation of the epithelial –mesenchymal
transition (EMT) program, and function in an
autocrine fashion to maintain the resulting
stem-cell state (Scheel et al. 2011). Thus, a com-
mon theme that emerges from these observa-
tions is that the outcome of signaling cross talk
is determined by the context of the signaling
environment and that multiple signal inputs,
rather than BMP or Wnt alone, are needed to
allow stem-cell fate determination (Kimelman
and Griffin 2000; Loose and Patient 2004). This
theme is frequently repeated in cross talk among
other pathways as well.
Cross Talk between TGF-b Family and Wnt
Signaling Occurs at Multiple Levels
On receptor activation, cross talk between TGF-
b family and Wnt signaling can occur at multi-
ple levels (Fig. 1).
Reciprocal Regulation of the Expression
of Pathway Ligands and Antagonists
Wnt signaling modulates the expression of
BMP or Nodal ligands, coreceptor or BMP an-
tagonists in embryos, adult stem cells, and can-
cer cells (Guo and Wang 2009), whereas BMP-2
and BMP-4 regulate the expression of Wnt-8 in
Xenopus (Hoppler and Moon 1998) or Wnt-7c
in chicken embryonic mesenchymal cells (Jin
et al. 2006). These regulations are likely to be
critical for establishing proper morphogen gra-
dients during cell-fate determination.
Cross Talk between Smads and Other Pathways
Advanced Online Article. Cite this article as Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a022137 3
on August 25, 2022 - Published by Cold Spring Harbor Laboratory Press http://cshperspectives.cshlp.org/Downloaded from
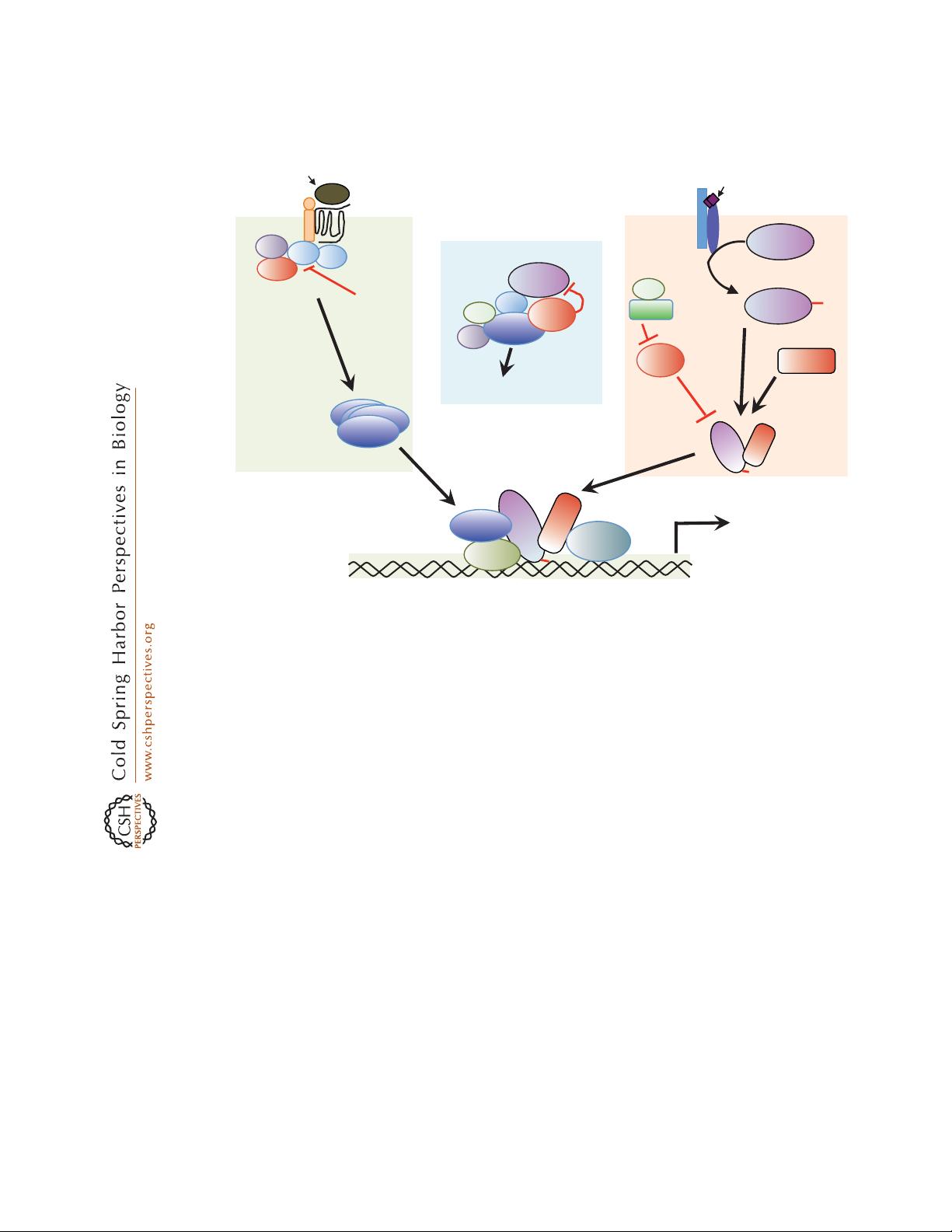
Direct Physical Interaction between and
Modification of Key Components of the Two
Pathways in the Cytoplasm and/or Nucleus
A well-documented mechanism of Smad regu-
lation by Wnt signaling is through phosphory-
lation of Smad proteins in the linker region by
GSK-3b (Fuentealba et al. 2007; Millet et al.
2009; Aragon et al. 2011). In mammalian cells
and Xenopus embryos, in the absence of Wnt,
GSK-3b phosphorylates the linker region of
Smad1, resulting in its polyubiquitylation and
degradation. Wnt signaling inhibits GSK-3b ac-
tivity and prevents Smad1 linker phosphoryla-
tion, leading to Smad1 stabilization (Fuentealba
et al. 2007; Aragon et al. 2011). Similarly, GSK-
3b phosphorylates Smad3 in the linker region
on Ser204, and this phosphorylation appears to
inhibit the transcription activity of Smad3. Mu-
tation of Ser204 to alanine strengthens the in-
teraction of Smad3 with transcription coactiva-
tors, and promotes its abilit y to activate target
genes and its ability to induce cell-cycle arrest
(Millet et al. 2009; Wang et al. 2009a). In the
absence of TGF-b, Axin and GSK-3b can bind
to Smad3 to promote its degradation. GSK-3b
β-Catenin
β-Catenin
β-Catenin
P
LEF/TCF
Cofactors
Developmental genes
Cell fate genes
TGF-β/BMP
R-Smad
R-Smad
P
Smad4
P
Smad7
APC
GSK-3
Axin
CK1
!
Smad3
P
Degradation
Unstimulated
Wnt
LRP
Dvl
Axin
GSK-3
CK1
Smurf1/2
Wnt
β-Catenin
β-Catenin
β-Catenin
Smad4
Smad4
R-Smad
R-Smad
GSK-3
APC
Figure 1. Cross talk between the transforming growth factor b (TGF-b) family and Wnt signaling at multiple
points. In the absence of TGF-b stimulation (middle), Smad3 can form a complex with Axin and glycogen
synthase kinase (GSK)-3b, where it is phosphorylated by GSK-3b, leading to its degradation. In the presence of
TGF-b or bone morphogenic proteins (BMPs) stimulation (right), GSK-3b also phosphorylates the activated
R-Smads (Smad1 or Smad3) in the linker region to inhibit their activity and promote degradation. Wnt
signaling inhibits GSK-3b and stabilizes the Smad proteins. Other components of the TGF-b pathway, including
Smurf1, Smurf2, and Smad7, also modulate Wnt signaling. In response to stimulation by Wnt, the canonical
Wnt pathway and the Smad pathway can synergize to activate transcription of target genes. Smad3 facilitates
b-catenin nuclear translocation and coordinates with the complex of b-catenin and T-cell factor (TCF) or
lymphoid enhancer–binding factor 1 (LEF1) at regulatory promoter sequences of target genes that contain
TCF- or LEF1-binding sites and/or Smad-binding sequences to regulate gene expression.
K. Luo
4
Advanced Online Article. Cite this article as Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a022137
on August 25, 2022 - Published by Cold Spring Harbor Laboratory Press http://cshperspectives.cshlp.org/Downloaded from

phosphorylates Smad3 at Thr66, leading to its
ubiquitylation and degradation, and this phos-
phorylation is further enhanced in the presence
of Axin. Through this linker phosphorylation,
Wnt signaling can control the basal level of
Smad3 activity in cells (Guo et al. 2008).
GSK-3b phosphorylation of Smad1 or
Smad3 appears to be a critical step in the se-
quential regulation of Smad activation and
subsequent destruction in response to BMP or
TGF-b and Wnt signals. Smad proteins are first
activated by BMP or TGF-b signaling through
phosphorylation at two carboxy-terminal ser-
ines. This activation is followed by a series of
phosphorylation events at the linker region
that is mediated by extracellular signal-regulat-
ed kinase (Erk) or p38 MAP kinases, or cyclin-
dependent kinase (CDK)8 or CDK9, which
prime the Smad proteins for binding to and
phosphorylation by GSK-3b (Fuentealba et al.
2007; Aragon et al. 2011). The regulation of
Smad proteins by GSK-3b in the presence of
BMP or TGF-b signals not only serves to inac-
tivate Smad signaling, but also provides a path
for the Wnt ligand to directly regulate Smad
activity. In vivo epistatic experiments in Xeno-
pus embryos indicate that Smad1 phosphoryla-
tion by GSK-3b plays a key role in mediating the
effects of Wnt signaling on neural development
at the gastrula stage and in ectodermal cells.
Furthermore, overexpression of Wnt-8 induced
epidermal differentiation dependent on activa-
tion of Smad1, 5, and/or 8 by BMP (Fuentealba
et al. 2007).
Negative regulation of Smad activity
through linker phosphorylation by GSK-3b
has also been observed in Drosophila (Eivers et
al. 2009, 2011; Quijano et al. 2011). In Drosoph-
ila, Mad is capable of signaling in both the Dpp
(BMP subfamily) and Wingless (Wnt family)
pathways, and the pathway choice depends on
the phosphorylation state of Mad. Signaling
downstream of Dpp requires the carboxy-ter-
minal phosphorylation of Mad, whereas un-
phosphorylated Mad participates in canonical
Wingless signaling to restrict self-renewing
mitosis by interacting with the transcription
factors Armadillo and Pangolin (homologs of
b-catenin and TCF, respectively). Both Wingless
and Dpp-induced functions of Mad are termi-
nated by GSK-3b-dependent linker phosphor-
ylation. Thus, Drosophila Mad can exist in three
functional states depending on the phosphory-
lation status. Given the conservation of Zw3/
GSK-3b phosphorylation sites in vertebrate
Smad1, 5, and 8, it is possible that this triphasic
response to Wingless- and TGF-b family- or
BMP-dependent Smad phosphorylation may
also be conserved during vertebrate embryonic
development (Shimmi and Newfeld 2013).
Smad proteins and Wnt pathway compo-
nents can also physically interact to regulate
the activity of each other (Fig. 1). Smad3 has
been found in the same complex as Axin and
CKI
1
, and GSK-3b in transfected cells as well as
human mesenchymal stem cells (MSCs), in the
absence of TGF-b stimulation in which Smad3
can be phosphorylated and inhibited by CKI
1
or GSK-3b (Furuhashi et al. 2001; Waddell
et al. 2004; Jian et al. 2006). The interaction of
Axin and Smad3 appears to facilitate the phos-
phorylation of Smad3 by the active TGF-b type
I receptor (TbRI) kinase, resulting in enhanced
transcriptional activation of reporter constructs
(Furuhashi et al. 2001). Smad3 also plays an
essential role in shuttling b-catenin into the
nucleus, likely through TGF-b-induced phos-
phorylation of Smad3 and the subsequen t re-
duction in the interaction of Smad3 with GSK-
3b (Jian et al. 2006). Dissociation of this protein
complex allows cotranslocation of b-catenin
and Smad3 into the nucleus, with Smad3 acting
as a chaperone, and this regulation is required
for the stimulation of MSC proliferation and
inhibition of MSC osteogenic differentiation
by TGF-b1.
Other positive and negative regulators of
the Smad pathway can also mediate cross talk
with the canonical Wnt pathway. For example,
Smurf1 and Smurf2 have been shown to inhibit
Wnt signaling by targeting Axin for ubiquityla-
tion, but using distinct mechanisms and with
different consequences. Smurf2 induces poly-
ubiquitylation of Axin at Lys505, leading to its
degradation (Kim and Jho 2010). Reducing en-
dogenous Smurf2 levels results in accumulation
of Axin and a subsequent decrease in b-catenin
signaling. Smurf1, on the other hand, ubiqui-
Cross Talk between Smads and Other Pathways
Advanced Online Article. Cite this article as Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a022137 5
on August 25, 2022 - Published by Cold Spring Harbor Laboratory Press http://cshperspectives.cshlp.org/Downloaded from