A high specific capacity membraneless aluminum-air cell operated with an inorganic/organic hybrid electrolyte
TLDR
In this paper, a microfluidic aluminum-air cell working with KOH methanol-based anolyte was developed in order to overcome the self-discharge issue of aluminum.About:
This article is published in Journal of Power Sources.The article was published on 2016-12-30 and is currently open access. It has received 12 citations till now. The article focuses on the topics: Electrolyte.read more
Figures

Figure 2 (a) Cell performance curves and (b) individual electrode polarization curves of 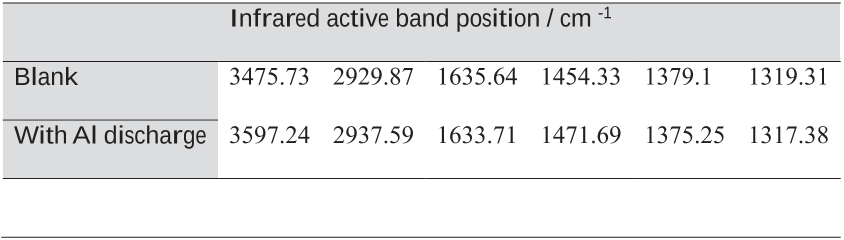
Table 3 Positions of FTIR characteristic bands shown in Figure 7. 
Figure 7 FTIR spectra of the powder samples obtained from KOH methanol-based solution without/with Al discharge. 
Table 2 Fitting results of EIS curves in Figure 4. 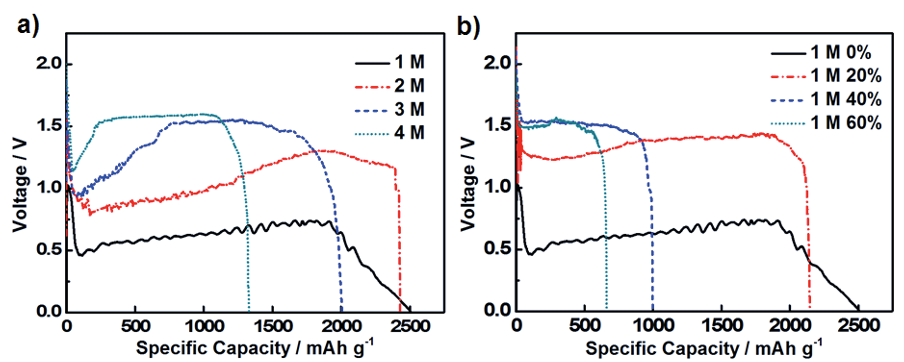
Figure 3 Specific capacities of Al foil in the hybrid electrolyte Al-air cells with (a) anolyte: neat methanol-based KOH solutions with KOH concentrations of 1 M, 2 M, 3 M and 4 M; 
Figure 5 SEM images of Al surface morphologies after discharging in (a) neat methanolbased and (b) water-based KOH anolyte. Insets are the corresponding sectional views of the Al electrodes.
Citations
More filters
Journal ArticleDOI
Recent advances and challenges in divalent and multivalent metal electrodes for metal–air batteries
Yangting Sun,Xiaorui Liu,Yiming Jiang,Jin Li,Jia Ding,Wenbin Hu,Wenbin Hu,Cheng Zhong,Cheng Zhong +8 more
TL;DR: In this article, different types of MABs are overviewed from the perspective of the metal electrodes, and the advantages and disadvantages of each system are presented, and recent advances that address challenges such as corrosion, passivation and dendrite growth are introduced.
Journal ArticleDOI
High-Performance and Recyclable Al-Air Coin Cells Based on Eco-friendly Chitosan Hydrogel Membranes.
Yisi Liu,Yisi Liu,Qian Sun,Xiaofei Yang,Xiaofei Yang,Jianneng Liang,Biqiong Wang,Alicia Koo,Ruying Li,Jie Li,Xueliang Sun +10 more
TL;DR: It is demonstrated that the presented Al-air coin cell can be recycled by a series of eco-friendly procedures using food-grade ingredients, resulting in recycled products that are environmentally safe and ready for reuse.
Journal ArticleDOI
Microfluidics for Electrochemical Energy Conversion
TL;DR: In this article , the authors present a review of the best practices in the field of microfluidic energy conversion for the past 20 years and present opportunities for future research directions and technology advances.
Journal ArticleDOI
Influences of L-Cysteine/Zinc Oxide Additive on the Electrochemical Behavior of Pure Aluminum in Alkaline Solution
References
More filters
Journal ArticleDOI
Metal–Air Batteries with High Energy Density: Li–Air versus Zn–Air
TL;DR: Li-air and Zn-air batteries have been studied extensively in the past decade as mentioned in this paper, with the aim of providing a better understanding of the new electrochemical systems, and metal-air battery with conversion chemistry is a promising candidate.
Journal ArticleDOI
Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuel cells
Frédéric Jaouen,Eric Proietti,Michel Lefèvre,Régis Chenitz,Jean-Pol Dodelet,Gang Wu,Hoon T Chung,Christina Johnston,Piotr Zelenay +8 more
TL;DR: In this paper, the authors focus on the new synthesis methods that have led to these breakthroughs and analyze the improvements required from NPMC-based catalysts to match the performance of Pt-based cathodes, even at high current density.
Journal ArticleDOI
A Critical Review of Li/Air Batteries
Jake Christensen,Paul Albertus,Roel S. Sánchez-Carrera,Timm Lohmann,Boris Kozinsky,Ralf Liedtke,Jasim Ahmed,Aleksandar Kojic +7 more
TL;DR: In this paper, the authors discuss the most critical challenges to developing robust, high-energy Li/air batteries and suggest future research directions to understand and overcome these challenges and predict that Li-air batteries will primarily remain a research topic for the next several years.
Journal ArticleDOI
Advanced zinc-air batteries based on high-performance hybrid electrocatalysts
Yanguang Li,Ming Gong,Yongye Liang,Ju Feng,Ji-Eun Kim,Hailiang Wang,Guosong Hong,Bo Zhang,Hongjie Dai +8 more
TL;DR: Li et al. as mentioned in this paper constructed stable zinc-air batteries using novel catalysts for oxygen reduction and evolution reactions, but their realization is hampered by the lack of efficient and robust air catalysts.
Journal ArticleDOI
Aluminum as anode for energy storage and conversion: a review
Qingfeng Li,Niels J. Bjerrum +1 more
TL;DR: In this paper, a review of aluminum-air secondary batteries is presented, including aqueous electrolyte primary batteries, aluminum air batteries, and molten salt secondary batteries, as well as solution additive to electrolytes.