A novel growth factor-dependent thermogenic brown adipocyte cell line from defined
precursor cells
Dagmar Kindler
1
*, Isabel S Sousa
1,2
*, Sabine Schweizer
3
*, Sarah Lerch
1
, Martin
Klingenspor
3,4,5
, Stephan Herzig
6,7
, Alexandros Vegiopoulos
1†
.
1 DKFZ Junior Group Metabolism and Stem Cell Plasticity, German Cancer Research
Center, Heidelberg 69120, Germany
2 Department of Life Sciences, University of Coimbra, Coimbra, Portugal
3 Chair for Molecular Nutritional Medicine, TUM School of Life Sciences, Technical
University of Munich, Freising, Germany
4 EKFZ – Else Kröner-Fresenius Center for Nutritional Medicine, Technical University of
Munich, Freising, Germany
5 ZIEL-Institute for Food and Health, Technical University of Munich, Freising, Germany
6 Helmholtz Center Munich, Institute for Diabetes and Cancer IDC, Neuherberg, Germany
7 Joint Heidelberg-IDC Translational Diabetes Program, Heidelberg University Hospital,
Heidelberg, Germany.
*Equal contribution
†
Corresponding author: Email: a.vegiopoulos@dkfz.de, vegiopoulos@web.de
Abstract
Molecular pathways regulating brown adipocyte formation and metabolism can be exploited
as targets for the treatment of obesity and disorders of glucose and lipid metabolism such as
type-2 diabetes. Investigations in this direction require adequate cell models for brown
adipocytes and their precursors. We report the establishment of a novel clonal cell line
derived from defined Lin
-
Sca1
+
adipocyte precursors from murine interscapular brown fat. In
contrast to most currently available lines, immortalization was achieved by serial passaging
without viral or genetic manipulation. Instead, the media were supplemented with basic
fibroblast growth factor, which was required for the maintenance of stable long-term growth
and immature morphology. BATkl2 cells differentiated to adipocytes with high efficiency
upon standard adipogenic induction independently of PPARg agonists and even at higher
passage numbers. BATkl2 adipocytes showed readily detectable Uncoupling protein 1
(Ucp1) protein expression and acutely responded to norepinephrine with increased Ucp1
mRNA expression, lipolysis and uncoupled mitochondrial respiration. Highly efficient siRNA-
mediated knockdown was demonstrated in the growth state as well as in differentiating
adipocytes, whereas plasmid DNA transfection was achieved in immature cells. These
features make the BATkl2 cell line an attractive brown (pre)-adipocyte cell model.
.CC-BY 4.0 International licenseavailable under a
not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (which wasthis version posted March 1, 2019. ; https://doi.org/10.1101/565168doi: bioRxiv preprint

Introduction
Brown adipose tissue (BAT) is a major contributor to adaptive thermogenesis in mammals
including humans and rodents. Its central function is the generation of heat through the
combustion of chemical energy (Cannon et al. 2004, Diaz et al. 2014, Sidossis et al. 2015).
In this way it can have a substantial contribution to organismal energy expenditure and
thereby influence energy balance in the long-term. The amount and activity of BAT depends
on environmental conditions, mainly temperature, as well as feeding status, age and obesity
(Diaz et al. 2014, Li et al. 2014). Evidence from rodent models and human studies has
established the protective function of BAT against obesity, the dysfunction of systemic
glucose and lipid metabolism and associated diseases such as type-2 diabetes. Thus,
enhancement of BAT function has become an actively pursued approach in the development
of new therapies in this area (Betz et al. 2018, Moonen et al. 2019, Sidossis et al. 2015). In
this direction it is critical to better understand the molecular regulation of BAT formation,
maintenance and metabolism.
BAT develops mostly before birth and is located in several confined and species-specific
anatomical sites (Diaz et al. 2014, Wang et al. 2016). The cell type responsible for heat
generation is the brown adipocyte, characterized by multilocular lipid droplets, high
mitochondrial content and high expression of the Uncoupling protein 1 (Ucp1). Although
alternative mechanisms have been demonstrated, Ucp1-mediated energy dissipation plays a
central role in heat generation (Cannon et al. 2004, Chouchani et al. 2019, Emont et al.
2019). This occurs through the uncoupling of the mitochondrial proton gradient and
respiration from ATP synthesis. The metabolism of brown adipocytes is optimized for the
efficient uptake and oxidation of substrates. This includes the release of intracellular fatty
acids by lipolysis. Metabolic activation including lipolysis and mitochondrial uncoupling are
controlled by various extracellular signals (Cannon et al. 2004, Emont et al. 2019). The major
physiological stimulus is norepinephrine, which is locally released by the sympathetic
nervous system.
Brown adipocytes are generated through the differentiation of immature mesenchymal
precursor cells, also termed brown preadipocytes (Wang et al. 2016). This process involves
lipid accumulation and mitochondrial biosynthesis and depends on the induction of a specific
gene expression program including Ucp1 protein expression. Brown adipocyte formation is
important beyond development and cell turnover, namely during the expansion of BAT upon
prolonged cold adaptation. In this case, the proliferation of precursor cells contributes to
increased capacity to form brown adipocytes (Lee et al. 2015, Nedergaard et al. 2019). BAT
precursor cells with the capacity to form brown adipocytes have been identified by lineage
tracing and flow cytometry in mice as Pdgfra
+
Sca1
+
cells (Lee et al. 2015). We have shown
that Lin(TER119/CD31/Cd45)
-
CD29
+
CD34
+
Sca1
+
cells from murine interscapular brown fat
efficiently form adipocytes ex vivo with a clearly distinct brown adipocyte expression profile
compared to their counterparts from white adipose tissue (Bayindir et al. 2015, Rodeheffer et
al. 2008).
The investigation of the molecular and biochemical mechanisms regulating precursor cell
proliferation and differentiation as well as brown adipocyte metabolism requires appropriate
cell culture models. Primary cells from the respective brown fat depots represent the most
faithful approach. However, primary cells are only available in relatively low numbers and
.CC-BY 4.0 International licenseavailable under a
not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (which wasthis version posted March 1, 2019. ; https://doi.org/10.1101/565168doi: bioRxiv preprint

have limited capacity for proliferative expansion in culture. Human BAT is poorly accessible
and only available from limited cohorts of patients. Primary cells can be functionally
expanded for a limited number of passages upon viral transduction with oncogenes.
Furthermore, several murine and human cell lines have been established through
immortalization by stable expression of viral oncogenes or telomerase subunits (Table 1)
(Cannon et al. 2001, Hirschberg et al. 2011, Klein et al. 1999). These preadipocytes can be
induced to differentiate to brown adipocytes in culture. However, the expression of
oncogenes and cell cycle regulators can confound conclusions on the regulation of precursor
proliferation and can interfere with differentiation. In addition, currently available cell lines
can have limitations such as the requirement for certain inducers for differentiation, limited
capacity for differentiation in the long-term or partial lack of key brown adipocyte features.
We sought to establish a stable brown adipocyte precursor cell line from a defined primary
cell population and without the use of viral/genetic manipulation. To this end we applied the
3T3 immortalization method with the addition of a physiological growth factor on primary
precursor cells isolated from murine interscapular brown fat.
Table 1. Overview of established murine and human brown preadipocyte cell lines
Cell line Origin Immortalization References
PAZ6
Human infant BAT
Viral oncogenes (SV40)
(Kazantzis et al.
2012, Zilberfarb et al.
1997)
hTERT-A41hBAT-SVF
Human deep neck fat
Telomerase (TERT)
(Xue et al. 2015)
Brown preadipocyte 1-3
Human supraclavicular fat
Viral oncogene (SV40)
(Shinoda et al. 2015)
TERT-hBA
Human deep neck fat
Telomerase (TERT)
(Markussen et al.
2017)
BFC-1
Mouse interscapular fat
Serial passage
(Forest et al. 1987)
HIB 1B
Mouse hibernoma
Viral oncogene (SV40)
(Ross et al. 1992)
HB2
Mouse interscapular fat
p53 knockout
(Irie et al. 1999)
WT-1
Mouse interscapular fat
Viral oncogene (SV40)
(Klein et al. 1999)
T37i
Mouse hibernoma
Viral oncogene (SV40)
(Penfornis et al.
2000)
Results
Establishment of a stably growing bFGF-dependent clone from interscapular brown adipose
tissue Lin
-
Sca1
+
precursor cells
With the aim of obtaining a defined suitable cell population for the immortalization of brown
adipocyte precursor cells, we isolated Lin
-
Sca1
+
cells from murine interscapular brown
.CC-BY 4.0 International licenseavailable under a
not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (which wasthis version posted March 1, 2019. ; https://doi.org/10.1101/565168doi: bioRxiv preprint

adipose tissue by magnetic separation. As described previously, this population represents a
good approximation of the Lin
-
CD29
+
CD34
+
Sca1
+
precursor/progenitor population (Babaei et
al. 2018, Bayindir et al. 2015). To apply the 3T3 immortalization approach we serially
passaged the cells over several weeks (Wu et al. 2012). However, we included basic
fibroblast growth factor (bFGF) in the media, which has been shown to promote
mesenchymal/fibroblastic cell proliferation and the maintenance of adipogenic differentiation
capacity (Hebert et al. 2009, Marquez et al. 2017, Widberg et al. 2009). The cultures
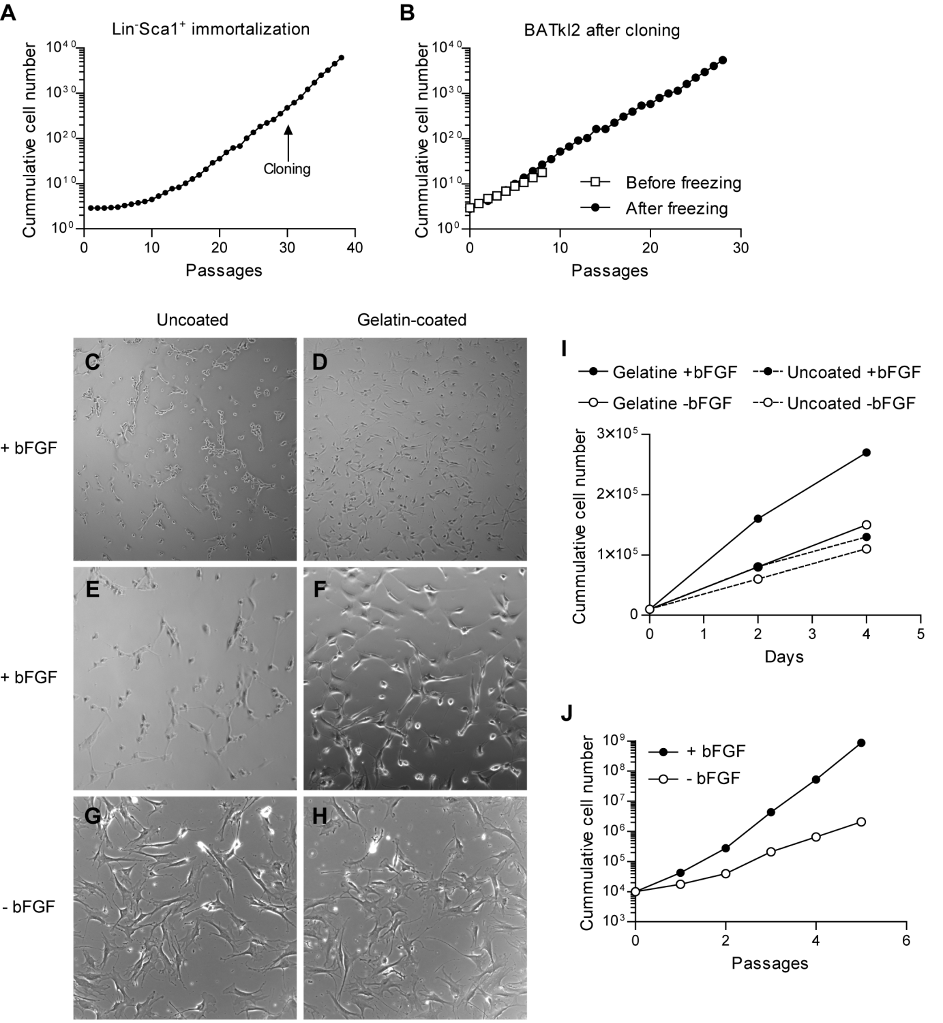
reached stable exponential growth by approximately 15 passages and cloning was
performed by serial dilution at 31 passages (Figure 1A). The clone designated BATkl2
showed stable exponential growth in the presence of bFGF, which was maintained for over
25 passages after a freeze-thaw cycle (Figure 1B). Notably, BATkl2 displayed poor
adherence to the standard tissue culture coating, resulting in loss of cells. Therefore, the
cells were transferred and permanently grown on gelatin-coated dishes, which resulted in
improved plastic adhesion, reduced intercellular adhesion and increased growth, at least in
the presence of bFGF (Figure 1C-I). The continuous culture with bFGF was associated with
a denser, more immature cell morphology and higher growth rate (Figure 1D,F,H-J).
Efficient brown adipogenic differentiation of BATkl2 cells
BATkl2 cultures were induced to differentiate with a standard adipogenic cocktail on gelatin-
coated dishes. Widespread adipogenic differentiation could be observed at 8 days after
induction (Figure 2A,C,D). Differentiation failed in the absence of gelatin coating due to poor
adhesion and loss of differentiating adipocytes (data not shown). Uncoupling protein 1
(Ucp1) is the prime expression marker for brown adipocytes and key mediator of the
mitochondrial uncoupling process. Differentiated BATkl2 cells had 1000-fold higher Ucp1
mRNA expression levels compared to non-induced BATkl2 cultures (Figure 2G). Ucp1
expression could be further elevated by acute treatment with norepinephrine (Figure 2G).
Importantly, Ucp1 protein could be robustly detected by Western blotting in differentiated but
not in undifferentiated BATkl2 cultures (Figure 2H). Addition of rosiglitazone during the first 2
days of differentiation resulted in slightly increased adipogenic differentiation and potentiated
Ucp1 expression by more than 10-fold (Figure 2A-G). However, Ucp1 mRNA levels were not
sensitive to norepinephrine treatment in cultures induced with rosiglitazone. Irrespective of
rosiglitazone treatment, the differentiation capacity and norepinephrine responsiveness were
maintained for at least 10-15 passages after replating of cryopreserved cells, when
passaging at the recommended low densities (data not shown).
Norepinephrine-dependent lipolysis and uncoupled mitochondrial respiration in BATkl2
adipocytes
To determine the capacity of BATkl2 differentiated adipocytes to respond metabolically to
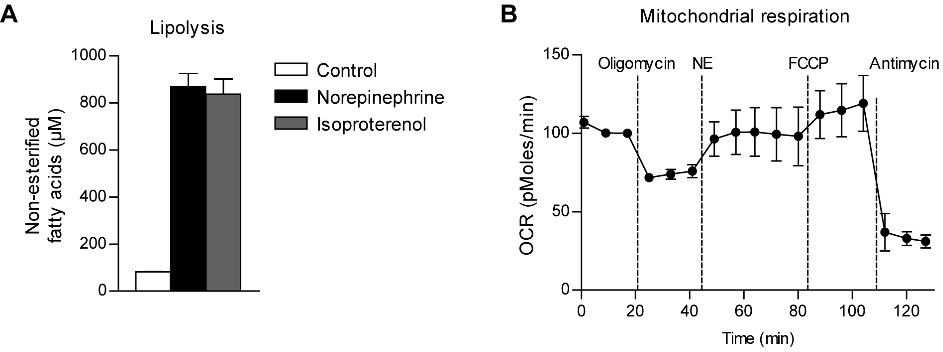
norepinephrine and beta-adrenergic stimulation, we measured the lipolysis-dependent
release of non-esterified fatty acids (NEFA). Treatment of differentiated BATkl2 cultures with
0.5 µM norepinephrine for 3 hours caused an approximately 10-fold increase in the
concentration of NEFA in the culture supernatants (Figure 3A), implying a potent activation
of triglyceride lipolysis. This effect could be recapitulated by treatment with 10 µM of the β-
adrenergic agonist isoproterenol (Figure 3A). Furthermore, we asked whether BATkl2
adipocytes were able to activate uncoupled mitochondrial respiration in response to
norepinephrine. To this end we differentiated BATkl2 cells in gelatin-treated XF96
microplates and measured their oxygen consumption rate (Seahorse analysis). BSA was
included in the media to reduce non-specific effects of lipolysis-derived NEFAs on
.CC-BY 4.0 International licenseavailable under a
not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (which wasthis version posted March 1, 2019. ; https://doi.org/10.1101/565168doi: bioRxiv preprint

mitochondrial respiration (Li et al. 2014). Treatment with oligomycin inhibits ATP synthase
and reveals the rate of uncoupled respiration. Stimulation with norepinephrine in the
presence of oligomycin acutely increased oxygen consumption to a substantial proportion of
the maximal respiratory capacity, determined by treatment with the general uncoupler FCCP
(Figure 3B).The data suggest that BATkl2 differentiated adipocytes respond to
norepinephrine by activating lipolysis and uncoupled mitochondrial respiration, representing
central metabolic functions of brown adipocytes.
Transfection efficiency in BATkl2 cells: siRNA and plasmid-mediated expression
We next tested whether BATkl2 cells were amenable to RNA interference using simple
siRNA transfection. Knockdown experiments in the undifferentiated state are relevant for the
investigation of gene function in precursor cell proliferation and early differentiation
processes. BATkl2 cells were transfected under general growth conditions with siRNA
targeting the Prkaca mRNA or with non-targeting siRNA (NC) and analyzed 2 days later.
Transfection with siNC caused an unspecific 2-fold increase in the expression of Prkaca
mRNA compared to non-transfected cells (Figure 4A). However, siPrkaca-transfected cells
displayed a 20-fold reduction in Prkaca expression compared to siNC, corresponding to
more than 90% knockdown efficiency (Figure 4A). The investigation of gene function in
adipocytes independently of any effects on precursor proliferation and differentiation requires
transfection after commitment to differentiation. To this end we transfected BATkl2 cells 5
days after the induction of differentiation with siPrkaca which resulted in approx. 90%
knockdown of Prkaca mRNA compared to siNC (Figure 4B). Finally, we tested the possibility
of transfecting BATkl2 cells with plasmid DNA in the general growing mode, aiming at the
overexpression of genes of interest. GFP-expressing cells were readily detectable by
fluorescence microscopy 24 hours after transfection with a GFP-encoding expression vector
(Figure 4C,D). The transfection efficiency was of intermediate level. In conclusion, BATkl2
cells were highly amenable to siRNA-mediated gene knockdown in the undifferentiated as
well as differentiated states and can in principle be transfected with DNA expression vectors.
Discussion
The novel BATkl2 cell line features a number of unique advantages compared to established
human and murine brown preadipocyte cell lines. (1) To our knowledge it is the first BAT-
derived cell line with defined cell type of origin, namely Lin
-
Sca1
+
cells representing
precursor cells. This can be of importance when it comes to investigations relevant to the
currently ill-defined cellular differentiation hierarchies in adipose tissue. (2) The key
distinguishing feature is that the immortalization process of BATkl2 cells is not dependent on
viral oncogenes or other exogenous cell cycle regulator genes. Most currently available cell
lines and immortalization protocols rely on such genes (Table 1) and this is a disadvantage
given the ability of oncogenes to directly and constitutively influence cell proliferation and
adipogenic differentiation. For instance, SV40 oncogenes have been shown to inhibit
adipogenesis (Cherington et al. 1988). The proliferation rate and immature cell morphology
of BATkl2 cells depended on continuous culture in the presence of the growth factor bFGF
even after more than 40 passages post isolation. Under these conditions BATkl2 showed
remarkably stable growth and maintained differentiation capacity at high passage number
and after cryopreservation. (3) BATkl2 showed high differentiation efficiency including Ucp1
protein expression without the need of treatment with agonists of the Peroxisome
Proliferator-Activated Receptor gamma (PPARγ). For instance, treatment with rosiglitazone
.CC-BY 4.0 International licenseavailable under a
not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (which wasthis version posted March 1, 2019. ; https://doi.org/10.1101/565168doi: bioRxiv preprint