
1
Endothelial Pannexin 1–TRPV4 channel signaling 1
lowers pulmonary arterial pressure 2
Panx1-TRPV4 signaling in pulmonary endothelium 3
4
1
Zdravka Daneva;
1,2
Matteo Ottolini;
1
Yen-Lin Chen;
1
Eliska Klimentova;
3
Soham A. Shah; 5
4
Richard D. Minshall;
5
Cheikh I. Seye,
6
Victor E. Laubach;
7
Brant E. Isakson;
1,7
Swapnil K. 6
Sonkusare 7
8
1
Robert M. Berne Cardiovascular Research Center, University of Virginia, Charlottesville, VA, 22908, 9
USA;
10
2
Department of Pharmacology, University of Virginia, Charlottesville, VA, 22908, USA; 11
3
Department of Biomedical Engineering, University of Virginia, Charlottesville, VA, 22908, USA; 12
4
Department of Anesthesiology, University of Illinois at Chicago, Chicago, IL, USA; Department of 13
Pharmacology, University of Illinois at Chicago, Chicago, IL, USA; 14
5
Department of Biochemistry, University of Missouri-Columbia, Columbia, MO, USA; 15
6
Department of Surgery, University of Virginia, Charlottesville, VA, 22908, USA; 16
7
Department of Molecular Physiology and Biological Physics, University of Virginia, Charlottesville, 17
VA, 22908, USA 18
19
20
Correspondence should be addressed to: 21
Swapnil K. Sonkusare, Ph.D. 22
University of Virginia School of Medicine 23
P.O. Box 801394 24
Charlottesville, VA 22908 25
E-mail: sks2n@virginia.edu 26
Phone: 434-297-7401 27
28
29
30
Key Words: Pannexin 1, TRP channels, pulmonary artery, endothelium, purinergic signaling, 31
caveolin 1. 32
33
.CC-BY 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted March 9, 2021. ; https://doi.org/10.1101/2021.03.09.434532doi: bioRxiv preprint

2
Abstract. 34
Pannexin 1 (Panx1) is an ATP-efflux channel that controls endothelial function in the systemic 35
circulation. However, the roles of endothelial Panx1 in resistance-sized pulmonary arteries (PAs) 36
are unknown. Extracellular ATP dilates PAs through activation of endothelial TRPV4 (transient 37
receptor potential vanilloid 4) ion channels. We hypothesized that endothelial Panx1–ATP–38
TRPV4 channel signaling promotes vasodilation and lowers pulmonary arterial pressure (PAP). 39
Endothelial, but not smooth muscle, knockout of Panx1 or TRPV4 increased PA contractility and 40
raised PAP. Panx1-effluxed extracellular ATP signaled through purinergic P2Y2 receptor 41
(P2Y2R) to activate protein kinase Cα (PKCα), which in turn activated endothelial TRPV4 42
channels. Finally, caveolin-1 provided a signaling scaffold for endothelial Panx1, P2Y2R, PKCα, 43
and TRPV4 channels in PAs, promoting their spatial proximity and enabling signaling interactions. 44
These results indicate that endothelial Panx1–P2Y2R–TRPV4 channel signaling, facilitated by 45
caveolin-1, reduces PA contractility and lowers PAP. 46
47
48
49
50
51
52
53
54
.CC-BY 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted March 9, 2021. ; https://doi.org/10.1101/2021.03.09.434532doi: bioRxiv preprint

3
Introduction 55
The pulmonary endothelium exerts a dilatory influence on small, resistance-sized 56
pulmonary arteries (PAs) and thereby lowers pulmonary arterial pressure (PAP). However, 57
endothelial signaling mechanisms that control PA contractility remain poorly understood. In this 58
regard, pannexin 1 (Panx1), which is expressed in the pulmonary endothelium and epithelium
1
, 59
has emerged as a crucial controller of endothelial function
2, 3
. Panx1, the most studied member of 60
the pannexin family, forms a hexameric transmembrane channel at the cell membrane that allows 61
efflux of ATP from the cytosol
4, 5
. Previous studies have indicated that Panx1
EC
promotes 62
endothelium-dependent dilation of systemic arteries
6, 7
, and endothelial cell (EC) Panx1 (Panx1
EC
) 63
has been linked to inflammation in pulmonary capillaries
8
. Beyond this, however, the 64
physiological roles of Panx1
EC
in the pulmonary vasculature are largely unknown. 65
Extracellular ATP (eATP) was recently shown to activate TRPV4 (transient receptor 66
potential vanilloid 4) channels in the endothelium of small PAs
9
, establishing endothelial TRPV4 67
(TRPV4
EC
) channels as potential signaling targets of Panx1
EC
in the pulmonary circulation. Ca
2+
68
influx through TRPV4
EC
channels is known to dilate small PAs through activation of endothelial 69
nitric oxide synthase (eNOS)
9
. These observations suggest that Panx1
EC
-released eATP may act 70
through TRPV4
EC
channels to reduce PA contractility and lower PAP. 71
Purinergic receptor signaling is an essential regulator of pulmonary vascular function
10-13
. 72
Previous studies in small PAs showed that eATP activates TRPV4
EC
channels through P2 73
purinergic receptors, although the precise P2 receptor subtype was not identified
9
. Pulmonary 74
endothelium expresses both P2Y and P2X receptor subtypes. Konduri et al. showed that eATP 75
dilates PAs through P2Y2 receptor (P2Y2R) activation and subsequent endothelial NO release
13
. 76
Recent evidence from systemic ECs and other cell types also supports P2Y2R-dependent 77
.CC-BY 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted March 9, 2021. ; https://doi.org/10.1101/2021.03.09.434532doi: bioRxiv preprint

4
activation of TRPV4 channels by eATP
14, 15
. These findings raise the possibility that the 78
endothelial P2Y2 receptor (P2Y2R
EC
) may be the signaling intermediate for Panx1
EC
–TRPV4
EC
79
channel communication in PAs. 80
The linkage between Panx1
EC
-mediated eATP release and subsequent activation of 81
P2Y2R
EC
–TRPV4
EC
signaling could depend on the spatial proximity of individual elements—82
Panx1
EC
, P2Y2R
EC
, and TRPV4
EC
—a functionality possibly provided by a signaling scaffold. 83
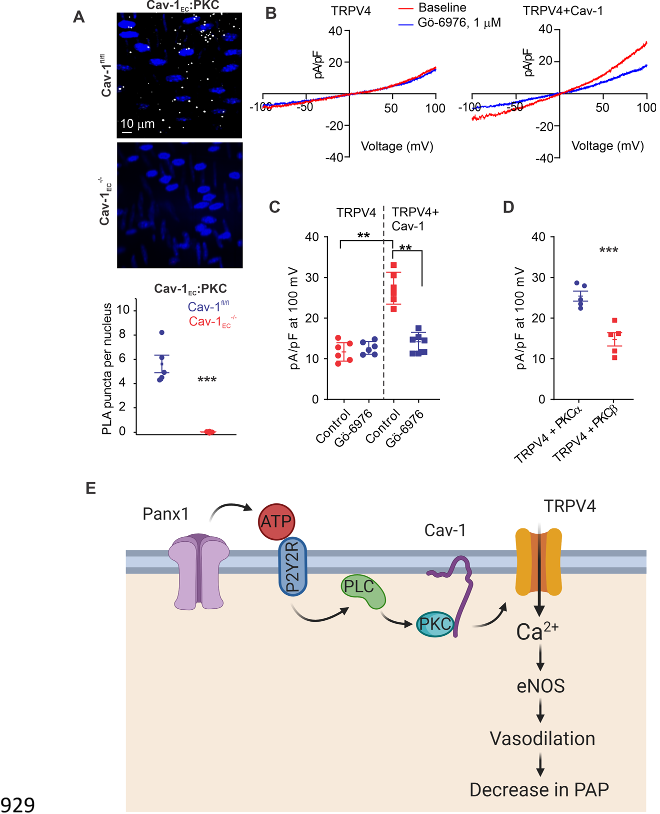
Caveolin-1 (Cav-1), a structural protein that interacts with and stabilizes other proteins in the 84
pulmonary circulation
16
, co-localizes with Panx1, P2Y2R, and TRPV4 channels in multiple cell 85
types
17-19
. Notably, global Cav-1
-/-
mice show elevated PAP, and endothelial Cav-1 (Cav-1
EC
)-86
dependent signaling is impaired in pulmonary hypertension
20-22
. 87
Here, we tested the hypothesis that Panx1
EC
–P2Y2R
EC
–TRPV4
EC
channel signaling, 88
supported by a signaling scaffold provided by Cav-1
EC
, reduces PA contractility and PAP. Using 89
inducible, EC-specific Panx1
-/-
, TRPV4
-/-
, P2Y2R
-/-
and Cav-1
EC
-/-
mice, we show that endothelial 90
Panx1–P2Y2R–TRPV4
signaling reduces PA contractility and lowers PAP. Panx1
EC
-generated 91
eATP acts via P2Y2R
EC
stimulation to activate protein kinase Cα (PKCα) and thereby increase 92
TRPV4
EC
channel activity. Panx1
EC
, P2Y2R
EC
, PKCα, and TRPV4
EC
channels co-localize with 93
Cav-1
EC,
ensuring spatial proximity among the individual elements and supporting signaling 94
interactions. Overall, these findings advance our understanding of endothelial mechanisms that 95
control PAP and suggest the possibility of targeting these mechanisms to lower PAP in pulmonary 96
vascular disorders. 97
98
99
.CC-BY 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted March 9, 2021. ; https://doi.org/10.1101/2021.03.09.434532doi: bioRxiv preprint

5
Results 100
Endothelial, but not smooth muscle, Panx1–TRPV4
signaling lowers PA contractility. 101
To clearly define the physiological roles of Panx1
EC
and TRPV4
EC
channels, we utilized 102
tamoxifen-inducible, EC-specific Panx1
EC
-/-
and TRPV4
EC
-/-
mice
23, 24
. Tamoxifen-injected 103
TRPV4
fl/fl
Cre
-
(TRPV4
fl/fl
) or Panx1
fl/fl
Cre
-
(Panx1
fl/fl
) mice were used as controls
8, 23
. 104
TRPV4
EC
-/-
mice showed elevated right ventricular systolic pressure (RVSP), a commonly used 105
in vivo indicator of PAP (Fig. 1A). In pressure myography experiments, ATP (1 µmol/L)-induced 106
dilation was absent in PAs from TRPV4
EC
-/-
mice (Fig. 1B), confirming that ATP dilates PAs 107
through TRPV4
EC
channels. RVSP was also elevated in Panx1
EC
-/-
mice (Fig. 1C). The Fulton 108
Index, a ratio of right ventricular (RV) weight to left ventricle plus septal (LV + S) weight, was 109
not altered in TRPV4
EC
-/-
or Panx1
EC
-/-
mice compared with the respective control mice, suggesting 110
a lack of right ventricular hypertrophy in these mice (Table 1). Importantly, baseline RVSP was 111
not altered in inducible, SMC-specific TRPV4 (TRPV4
SMC
-/-
) or Panx1 (Panx1
SMC
-/-
) knockout 112
mice (Fig. 1A and C). Functional cardiac MRI studies indicated no alterations in cardiac function 113
in TRPV4
EC
-/-
or Panx1
EC
-/-
mice compared with the respective control mice (Table 1), suggesting 114
that the changes in RVSP were not due to altered cardiac function. 115
Localized, unitary Ca
2+
influx signals through TRPV4
EC
channels, termed TRPV4
EC
116
sparklets
25
, were recorded in en face
,
4th-order PAs (~ 50 µm) loaded with Fluo-4. Baseline 117
TRPV4
EC
sparklet activity and activity induced by a low concentration (1 nmol/L) of the specific 118
TRPV4 channel agonist, GSK1016790A (hereafter, GSK101), were significantly reduced in PAs 119
from Panx1
EC
-/-
mice compared with those from Panx1
fl/fl
mice (Fig. 1D). Additionally, the number 120
of TRPV4
EC
sparklet sites per cell was decreased in PAs from Panx1
EC
-/-
mice (Fig. 1E). At a 121
.CC-BY 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted March 9, 2021. ; https://doi.org/10.1101/2021.03.09.434532doi: bioRxiv preprint




![Figure 2. eATP activates TRPV4EC channels via P2Y2REC stimulation. A, Release of ATP 842 (nmol/L) from PAs of Panx1fl/fl, Panx1EC-/-, TRPV4fl/fl, and TRPV4EC-/- mice (n = 5–6; *P < 0.05 843 vs. Panx1fl/fl; t-test]. B, left, Representative traces showing TRPV4EC sparklet activity in en face 844 preparations of PAs from Panx1fl/fl mice in the absence or presence of apyrase (10 U/mL). 845 Experiments were performed in Fluo-4–loaded PAs in the presence of CPA (20 µmol/L), included 846 to eliminate Ca2+ release from intracellular stores. Right, TRPV4EC sparklet activity (NPO) per site 847 in en face preparations of PAs from Panx1fl/fl and Panx1EC-/- mice in the presence or absence of 848](/figures/figure-2-eatp-activates-trpv4ec-channels-via-p2y2rec-2n4ni5qf.png)