REGEN-COV for COVID-19
1
1
2
Casirivimab and imdevimab in patients admitted to
3
hospital with COVID-19 (RECOVERY): a randomised,
4
controlled, open-label, platform trial
5
6
Running title: REGEN-COV for COVID-19
7
8
RECOVERY Collaborative Group*
9
10
11
*The writing committee and trial steering committee are listed at the end of this
12
manuscript and a complete list of collaborators in the Randomised Evaluation of
13
COVID-19 Therapy (RECOVERY) trial is provided in the Supplementary Appendix.
14
15
Correspondence to: Prof Peter W Horby and Prof Martin J Landray, RECOVERY Central
16
Coordinating Office, Richard Doll Building, Old Road Campus, Roosevelt Drive, Oxford OX3
17
7LF, United Kingdom.
18
Email: recoverytrial@ndph.ox.ac.uk
19
20
21
22
. CC-BY 4.0 International licenseIt is made available under a
is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. (which was not certified by peer review)
The copyright holder for this preprint this version posted June 16, 2021. ; https://doi.org/10.1101/2021.06.15.21258542doi: medRxiv preprint
NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice.

REGEN-COV for COVID-19
2
SUMMARY
23
Background: REGEN-COV
is a combination of 2 monoclonal antibodies (casirivimab
24
and imdevimab) that bind to two different sites on the receptor binding domain of the
25
SARS-CoV-2 spike protein. We aimed to evaluate the efficacy and safety of REGEN-
26
COV in patients admitted to hospital with COVID-19.
27
Methods: In this randomised, controlled, open-label platform trial, several possible
28
treatments were compared with usual care in patients hospitalised with COVID-19.
29
Eligible and consenting patients were randomly allocated (1:1) to either usual standard of
30
care alone (usual care group) or usual care plus a single dose of REGEN-COV 8g
31
(casirivimab 4g and imdevimab 4g) by intravenous infusion (REGEN-COV group). The
32
primary outcome was 28-day mortality assessed first among patients without detectable
33
antibodies to SARS-CoV-2 at randomisation (seronegative) and then in the overall
34
population. The trial is registered with ISRCTN (50189673) and clinicaltrials.gov
35
(NCT04381936).
36
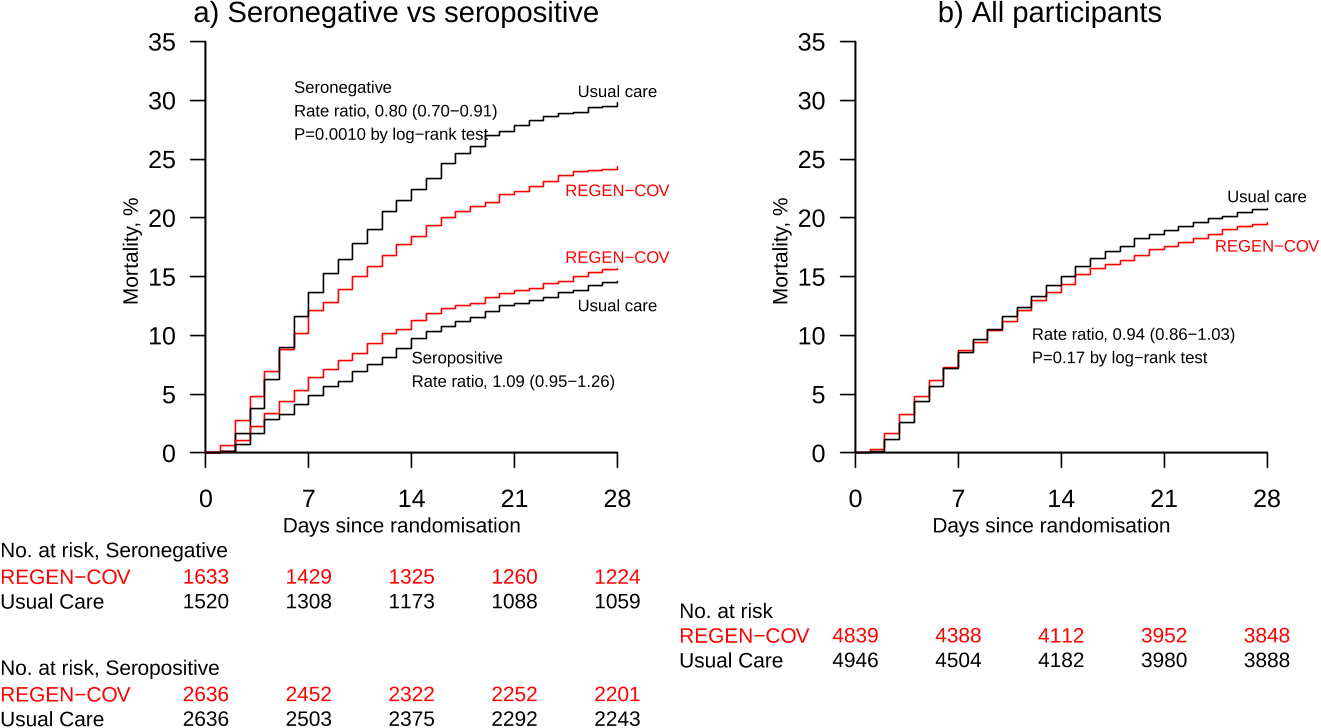
Findings: Between 18 September 2020 and 22 May 2021, 9785 patients were randomly
37
allocated to receive usual care plus REGEN-COV or usual care alone, including 3153
38
(32%) seronegative patients, 5272 (54%) seropositive patients and 1360 (14%) patients
39
with unknown baseline antibody status. In the primary efficacy population of seronegative
40
patients, 396 (24%) of 1633 patients allocated to REGEN-COV and 451 (30%) of 1520
41
patients allocated to usual care died within 28 days (rate ratio 0·80; 95% CI 0·70-0·91;
42
p=0·0010). In an analysis involving all randomised patients (regardless of baseline
43
antibody status), 944 (20%) of 4839 patients allocated to REGEN-COV and 1026 (21%)
44
of 4946 patients allocated to usual care died within 28 days (rate ratio 0·94; 95% CI 0·86-
45
. CC-BY 4.0 International licenseIt is made available under a
is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. (which was not certified by peer review)
The copyright holder for this preprint this version posted June 16, 2021. ; https://doi.org/10.1101/2021.06.15.21258542doi: medRxiv preprint

REGEN-COV for COVID-19
3
1·03; p=0·17). The proportional effect of REGEN-COV on mortality differed significantly
46
between seropositive and seronegative patients (p value for heterogeneity = 0·001).
47
Interpretation: In patients hospitalised with COVID-19, the monoclonal antibody
48
combination of casirivimab and imdevimab (REGEN-COV) reduced 28-day mortality
49
among patients who were seronegative at baseline.
50
Funding: UK Research and Innovation (Medical Research Council) and National Institute
51
of Health Research (Grant ref: MC_PC_19056).
52
Keywords: COVID-19, monoclonal antibodies, spike protein, clinical trial.
53
54
. CC-BY 4.0 International licenseIt is made available under a
is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. (which was not certified by peer review)
The copyright holder for this preprint this version posted June 16, 2021. ; https://doi.org/10.1101/2021.06.15.21258542doi: medRxiv preprint

REGEN-COV for COVID-19
4
INTRODUCTION
55
Monoclonal antibodies (mAbs) are a set of identical antibodies that have high specificity
56
and affinity for a single epitope. They have been demonstrated to be safe and effective in
57
selected viral diseases when used for prophylaxis (respiratory syncytial virus) or
58
treatment (Ebola virus disease).
1-3
The clinical efficacy of mAbs in viral infections is
59
thought to be mediated through direct binding to free virus particles and neutralisation of
60
their ability to infect host cells. mAbs may also bind to viral antigens expressed on the
61
surface of infected cells and stimulate antibody-dependent phagocytosis and cytotoxicity
62
via the Fc portion of the mAb.
4
63
SARS-CoV-2 infection is initiated by binding of the viral transmembrane spike
64
glycoprotein to angiotensin converting enzyme 2 (ACE2) on the surface of host cells.
5
65
The receptor binding domain of the spike glycoprotein is, consequently, the main target
66
for neutralising antibodies.
6
Following the emergence of SARS-COV-2, mAbs targeting
67
the spike receptor binding domain were rapidly isolated from humanised mice and from
68
peripheral B cells of recovered patients.
7,8
Anti-SARS-CoV-2 spike protein neutralizing
69
mAbs have demonstrated in vivo efficacy in both therapeutic and prophylactic settings in
70
mouse, and non-human primates models, with decreases in viral load and lung
71
pathology.
9-12
72
Regeneron Pharmaceuticals (Tarrytown, New York, USA) has developed two non-
73
competing, high-affinity human IgG1 anti-SARS-CoV-2 mAbs, casirivimab and
74
imdevimab, which bind specifically to the receptor binding domain of the spike
75
glycoprotein of SARS-CoV-2, blocking viral entry into host cells.
13
A combination of
76
. CC-BY 4.0 International licenseIt is made available under a
is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. (which was not certified by peer review)
The copyright holder for this preprint this version posted June 16, 2021. ; https://doi.org/10.1101/2021.06.15.21258542doi: medRxiv preprint

REGEN-COV for COVID-19
5
antibodies that bind to non-overlapping epitopes, rather than a single antibody, is
77
intended to minimize the likelihood of loss of antiviral activity due to naturally circulating
78
viral variants or development of escape mutants under drug pressure.
14
In a clinical study
79
in non-hospitalised adults with SARS-COV-2 infection and risk factors for severe COVID-
80
19, the combination of casirivimab and imdevimab (REGEN-COV) was safe and,
81
compared to placebo, reduced virus load in the upper airway, shortened the time to
82
symptom resolution, and reduced the composite outcome of COVID-19-related
83
hospitalisation or all-cause mortality.
15,16
Other anti-spike mAb products have also
84
demonstrated an antiviral and clinical effect in non-hospitalised adults with SARS-COV-
85
2 infection.
17,18
In the United States, Emergency Use Authorization has been given for the
86
use of bamlanivimab with etesevimab, REGEN-COV, and sotrovimab in non-hospitalised
87
patients with mild to moderate COVID-19. The European Medicines Agency has
88
authorised REGEN-COV for use in patients who are at high risk of progressing to severe
89
COVID-19 but do not require supplemental oxygen. Interim results from a small trial of
90
REGEN-COV in hospitalised patients requiring low-flow oxygen was consistent with a
91
clinical benefit in seronegative patients.
19
92
However, to date, no virus-directed therapy has been shown to reduce mortality in
93
hospitalised patients with COVID-19, for whom the only treatments so far shown to reduce
94
mortality have been those that modify the inflammatory response.
20-22
. The only published
95
trial of an anti-spike mAb (bamlanivimab) in hospitalised patients was terminated for
96
futility after 314 patients had been randomised.
23,24
Two other studies of mAb products
97
(VIR-7831 monotherapy, and BRII-196 with BRII-198 combination therapy) in
98
hospitalized COVID-19 patients were also terminated for futility with sample sizes of 344
99
. CC-BY 4.0 International licenseIt is made available under a
is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. (which was not certified by peer review)
The copyright holder for this preprint this version posted June 16, 2021. ; https://doi.org/10.1101/2021.06.15.21258542doi: medRxiv preprint