




read more










While antibody tests are recommended for seroprevalence and epidemiological studies, the “gold standard” among COVID-19 molecular tests is the quantitative reverse transcription-polymerase chain reaction (RT-qPCR) (5).
Given the rapid spread of the disease and the arrival of new pandemic waves, it remains urgent to implement early and massive SARS-CoV-2 testing strategies alongside with contact tracing and isolation (3).
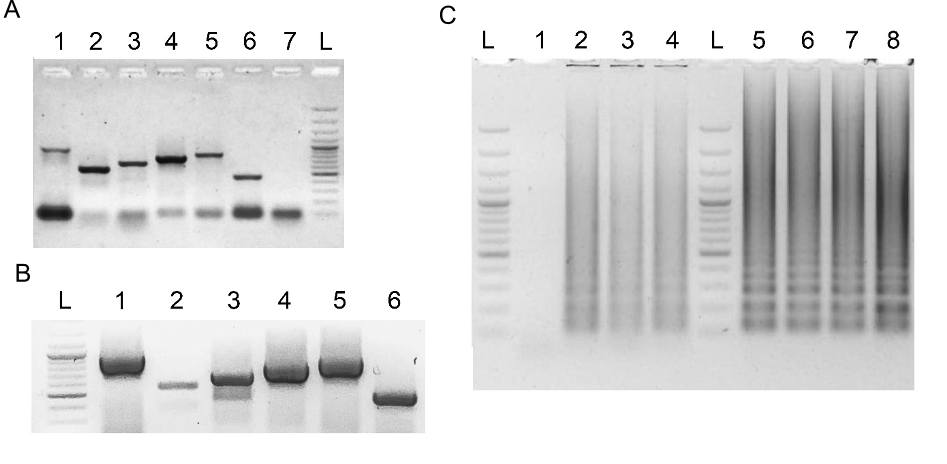
RCSMS routinely detected 50 copies per 10 µl reaction in triplicate assays, occasionally reaching five copies in one or two replicates.
In addition, seven incongruencies (2.8%) were recorded between the two tests: two false positives (TFP=2.6%) and five false negatives (TFN=2.5%).
A sharp decrease of PPV in low prevalence settings is common to all diagnostic tests because of the higher probability of obtaining false positives (31).
In sum, obtaining high-quality diagnostic data is essential to correctly monitor the evolution of the epidemic, ensure the success of public health strategies and the transition to a new normality.
Recognition of viral target sequences by the RNP complex triggers the collateral activity of Cas12a, resulting in the cleavage of ssDNA reporter probes.
For immunocromatographyic (qualitative) readout, a lateral flow strip is then inserted into the CRISPR-Cas12a reaction tube or well.
the NPV of diagnostic tests is expected to drop in high prevalence settings, as the number of false negatives increase (31).
Ensuring a low rate of false negatives (positive individuals being deemed negative) is especially desirable considering that COVID-19 is a serious, largely asymptomatic and contagious disease, for which early measures are advisable.
Ten individuals (3.6% of the total) did not record their date of symptom onset; therefore, their results were excluded from the analysis stratified by time of illness.
three rounds of analyses reproduced these results, with only two positive samples yielding a negative result in one of their three replicates.
their statistical simulation for RCSMS using the currently available data (276 out of 350 samples analyzed, Fig. 7), predicts excellent NPVs >95% for prevalence scenarios as low as 1% and as high as 50%.
The limit of detection (LOD) for both viral genes was determined using serial dilutions of the synthetic RNA templates, covering the range from 250 to 1 viral copy per 10 µl RT-LAMP reaction.
These PPV estimates are consistent with the results from their field study, where prevalence reached almost 30%, strengthening the conclusion that RCSMS is particularly well suited for the identification of positive cases during acute pandemic phases, such as the current second waves hitting Perú, India and Brazil.
the level of concordance (Cohen's Kappa coefficient) between RT-qPCR and RCSMS was calculated for both analytical and clinical validations, as well as sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), the area under the curve (AUC), accuracy of RCSMS, and PPV/ NPV simulations assuming various prevalence scenarios.